Diagnostics of soft tissue tumors

Univ.-Prof. Steffen U. Eisenhardt

Prof. Dr. David Braig

Dr. Anja Eisenhardt

Dr. Alexander Runkel

Dr. Adrian Schmid

Dr. Annika Schwacha

Sai Surendran
Liquid Biopsy in soft tissue sarcomas
Malignant soft tissue tumors (soft tissue sarcomas) are rare and highly diverse malignant tumors. Modern and reliable diagnosis is crucial for successful treatment. Our clinic is conducting intensive research into new, preferably non-invasive options - such as liquid biopsy. This involves searching for tumor components in a blood sample, which can complement procedures such as conventional tissue sampling and radiological imaging.
This is especially important in tumor follow-up as about 50% of soft tissue sarcomas relapse after treatment. Early detection and treatment of local recurrence or metastasis can significantly improve long-term disease control and survival. Most recurrences develop within 2-3 years after treatment. Closely spaced and efficient follow-up strategies are therefore mandatory to detect recurrence at an early stage.
Current follow-up strategies, which consist of a physical examination and various imaging modalities are expensive, time consuming, associated with radiation exposure, and cover only parts of the body.
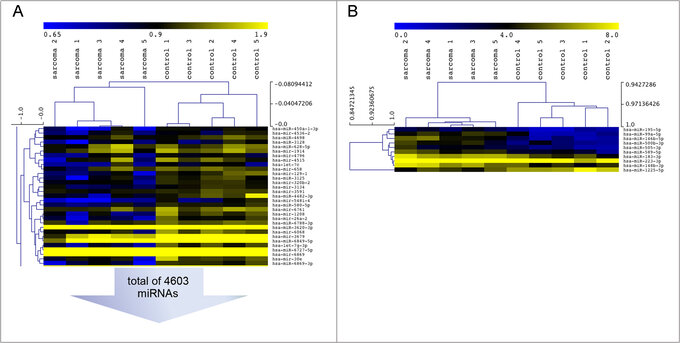
We want to distinguish patients with active disease from patients in complete remission by liquid biopsy. Different tumor-induced changes in the peripheral blood of patients with soft tissue sarcomas are targeted. We recently discovered a miRNA signature in the peripheral blood of patients with Synovial Sarcomas, which distinguished them from healthy persons and other sarcoma entities and established sensitive methods for the detection of sarcoma associated fusion genes.
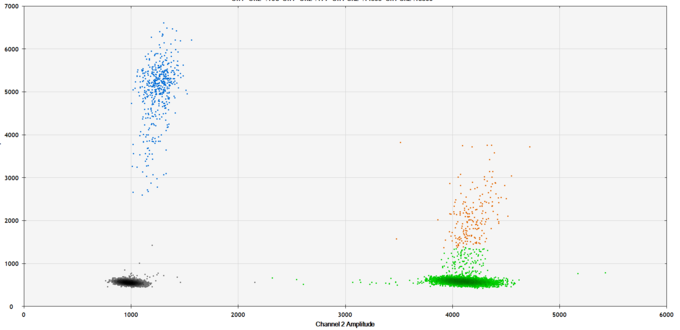
Present research focuses on the detection of free circulating tumor DNA (ctDNA). Highly sensitive methods enable the quantification of tumor-derived ctDNA in blood due to tumor-specific DNA alterations. In the absence of tumor tissue, no ctDNA is detectable. In the case of tumor recurrence, tumor-specific ctDNA can be quantified in the peripheral circulation, which increases with growing tumor burden. In contrast to imaging, this approach is independant of the anatomic location of the tumor and might even surpass current follow-up stategies.
ctDNA can also be used to monitor tumor treatment. It can support imaging to predict the response of radiation therapy or systemic therapy by repeatedly analyzing blood samples during treatment. This might help guide optimal, individualized tumor treatment.
FRKS002237: Analysis of biomarkers in the peripheral blood of patients with soft tissue sarcomas.
FRKS004314: Evaluation of the response of soft tissue sarcomas to neoadjuvant radiation therapy by quantifying circulating tumor DNA and multiparametric magnetic resonance imaging.
Schmid A, Lausch U, Runkel A, Kiefer J, Pauli T, Boerries M, Bogner B, Eisenhardt SU, Braig D. Improved Quantification of Circulating Tumor DNA in Translocation-Associated Myxoid Liposarcoma by Simultaneous Detection of Breakpoints and Single Nucleotide Variants. Cancer Med. 2025 Feb;14(4):e70704
Runkel A, Braig D, Bogner B, Schmid A, Lausch U, Boneberg A, Brugger Z, Eisenhardt A, Kiefer J, Pauli T, Boerries M, Fuellgraf H, Kurowski K, Bronsert P, Scholber J, Grosu AL, Rovedo P, Bamberg F, Eisenhardt SU, Jung M (2023). Non-invasive monitoring of neoadjuvant radiation therapy response in soft tissue sarcomas by multiparametric MRI and quantification of circulating tumor DNA - a study protocol. PLoS One. 2023 Nov 1;18(11):e0285580.
Braig D, Runkel A, Eisenhardt AE, Schmid A, Zeller J, Pauli T, Lausch U, Wehrle J, Bronsert P, Jung M, Kiefer J, Boerries M, Eisenhardt SU (2022). Individualized Mini-Panel Sequencing of ctDNA Allows Tumor Monitoring in Complex Karyotype Sarcomas. Int J Mol Sci. 2022 Sep 6;23(18):10215.
Eisenhardt AE, Brugger Z, Lausch U, Kiefer J, Zeller J, Runkel A, Schmid A, Bronsert P, Wehrle J, Leithner A, Liegl-Atzwanger B, Giunta R, Eisenhardt SU, Braig D (2022). Genotyping of circulating free DNA enables monitoring of tumor dynamics in Synovial Sarcomas. Cancers. 2022 Apr 21;14(9):2078.
Eisenhardt AE, Schmid A, Esser J, Brugger Z, Lausch U, Kiefer J, Braig M, Runkel A, Wehrle J, Claus R, Bronsert P, Leithner A, Liegl-Atzwanger B, Zeller J, Papini R, von Laffert M, Pfitzner BM, Koulaxouzidis G, Giunta RE, Eisenhardt SU, Braig D (2022). Targeted next-generation sequencing of circulating free DNA enables non-invasive tumor detection in myxoid liposarcomas. Molecular Cancer – 2022 Feb 14;21(1):50.
Braig D, Becherer C, Bickert C, Braig M, Claus R, Eisenhardt AE, Heinz J, Scholber J, Herget GW, Bronsert P, Fricke A, Follo M, Stark GB, Bannasch H, Eisenhardt SU. (2019). Genotyping of circulating cell-free DNA enables noninvasive tumor detection in myxoid liposarcomas. Int J Cancer. 2019 Aug 15;145(4):1148-1161.
Fricke A, Cimniak AFV, Ullrich PV, Becherer C, Bickert C, Pfeifer D, Heinz J, Stark GB, Bannasch H, Braig D, Eisenhardt SU (2018). Whole Blood miRNA Expression Analysis Reveals miR-3613-3p As A Potential Biomarker For Dedifferentiated Liposarcoma. Cancer Biomarkers. 2018 Apr 9. doi: 10.3233/CBM-170496.
Fricke A, Ullrich PV, Cimniak AF, Follo M, Nestel S, Heimrich B, Nazarenko I, Stark GB, Bannasch H, Braig D, Eisenhardt SU (2016). Synovial Sarcoma Microvesicles Harbor the SYT-SSX Fusion Gene Transcript: Comparison of Different Methods of Detection and Implications in Biomarker Research. Stem Cells International. 2016; 6146047.
Fricke A, Ullrich PV, Heinz J, Pfeifer D, Scholber J, Herget GW, Hauschild O, Bronsert P, Stark GB, Bannasch H, Eisenhardt SU, Braig D (2015). Identification of a blood-borne miRNA signature of synovial sarcoma. Molecular Cancer. 2015; 7;14:151.
Innovative biopsy strategies in soft-tissue tumors
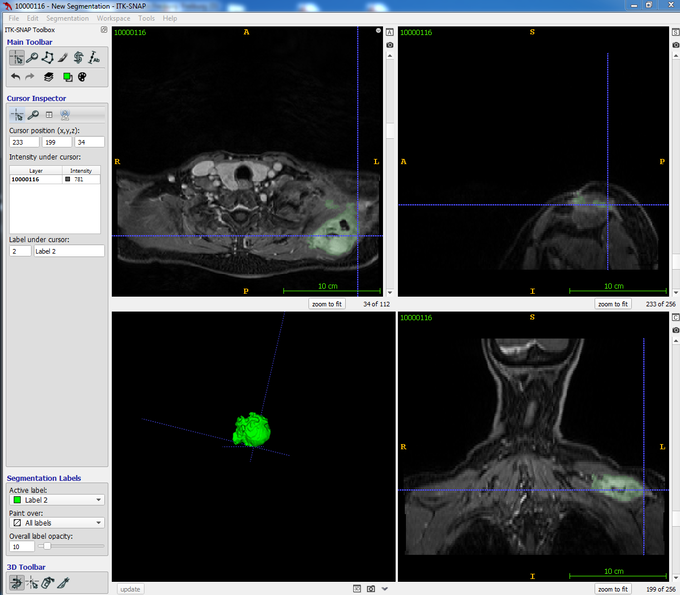
Progress has also been made in classic biopsies: new techniques allow samples to be taken with particular precision and care under ultrasound, CT, or MRI guidance, which increases the safety and quality of the findings. This research is conducted in collaboration with the Department of Radiology (Dr. Balazs Bogner).
Genetic heterogeneity in soft-tissue tumors
Another important focus of our interest is the genetic analysis of tumors. Methods such as spatially resolved next-generation sequencing (NGS) and transcriptome analysis can be used to determine the individual genetic characteristics of tumors. This helps us to better understand the great diversity and heterogeneity of these tumors and to plan customized therapies for each tumor in the future.
Schmid A, Eisenhardt AE, Bogner B, Runkel A, Lausch U, Pauli T, Antolini LN, Boneberg A, Kiefer J, Bronsert P, Boerries M, Eisenhardt SU, Braig D. Intratumoral heterogeneity of cancer driver genomic alterations in myxoid liposarcomas. Cancer. 2025 Jun 15;131(12):e35937.
Genetic sarcoma predisposition
Genetic predispositions can increase the risk of developing soft tissue tumors. This might influence tumor treatment, follow-up, but also tumor screening for other tumors that may occur due to genetic predisposition. In addition, tumor-causing germline mutations can be inherited, meaning that relatives may also be affected and appropriate screening can be offered. We offer patients with sarcomas a diagnostic study on genetic predisposition. This multicenter study is led by Prof. Hettmer (University Clinic and Polyclinic for Pediatrics I, Halle) and recruits children, adolescens and adults up to the age of 50.
FRKS005589: Sarcoma predisposition: Individual assessment of genetic susceptibility to sarcoma in children, adolescents, and young adults with soft tissue sarcomas.
This research lays the foundation for treating affected individuals early, individually, and as precise as possible.