Cardiovascular Imaging and Immunology
Projektleiter: Prof. Dr. Timo Heidt
Areas of interest
I - Cardiovascular Imaging
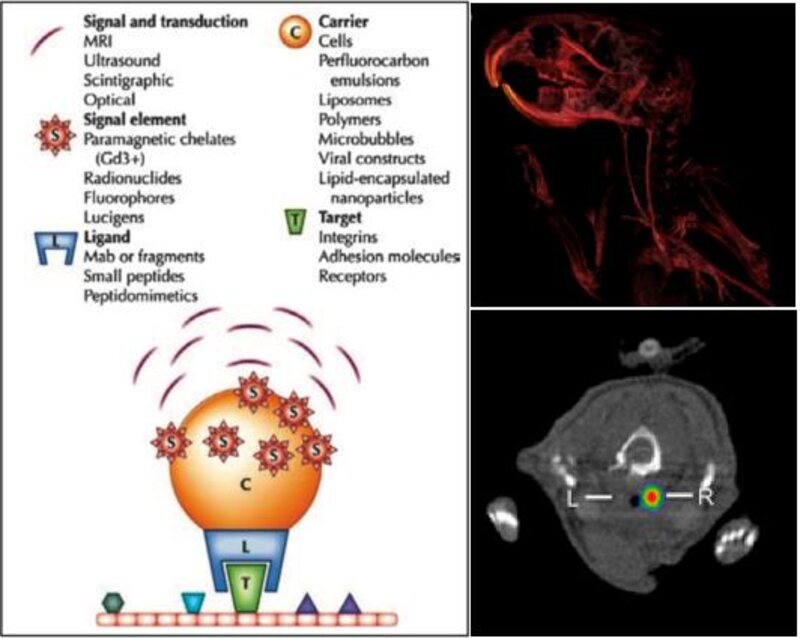
Non-invasive imaging using target specific contrast agents allows visualization of disease specific structures of interest, adding functional information and increasing sensitivity of detection. Targeted contrast agents consist of a specific ligand, either antibody or peptide, conjugated to a signal element, which can be a radionuclide, paramagnetic substrate or microbubble, subsequently used for PET/SPECT, magnetic resonance or ultrasound imaging. In our group, we are primarily interested in targeting activated platelets. Activated platelets are involved in atherosclerotic plaques progression. Especially during erosion or rupture of inflamed lesions, platelets are activated on the endothelial surface. Detection of areas with increased platelet activation could thus be facilitated to identify unstable coronary atherosclerotic plaques. Further cardiovascular diseases that involve platelet activation include myocardial infarction, pulmonary embolism and infective endocarditis. Non-invasive imaging of activated platelets may provide information about inflammation as well as extent and activity of disease. Our goal is to investigate how molecular cardiovascular imaging can be facilitated for diagnostics and clinical decision making to ultimately help the physician in guiding the patient.
Figure I Left Schematic construct of a targeted contrast agent. Conjugation of a contrast-giving element (e.g. gadolinium, iron oxide, radionuclide or microbubble) to a specific ligand (antibody or peptide) allows binding of contrast agents at cellular targets of interest. Right Example of a contrast agent application, showing the detection of a vascular thrombosis in the murine carotid artery. Above Contrast enhanced CT of the murine aorta and carotid arteries. Below Signal of a thrombosis in the right carotid artery after injection of an In-111-labelled contrast agent targeting activated platelets.
II - Cardiovascular Immunology
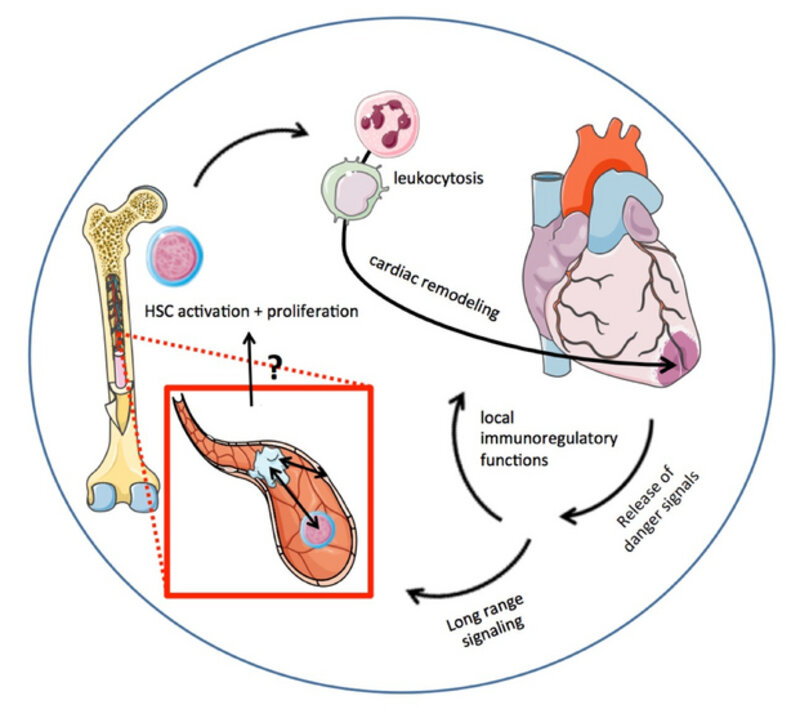
Innate immunity has been recognized to be an important player in wound healing and cardiac remodeling following myocardial infarction. After ischemic tissue injury, a burst of pro-inflammatory factors and cytokines is released from the infarct tissue into the blood to trigger recruitment of inflammatory immune cells (e.g. neutrophils, monocyte/macrophages). These immune cells locally orchestrate break down and removal of necrotic tissue as well as wound healing and tissue remodeling. For this reason, inflammation is an indispensable requirement for adequate myocardial recovery. On the other hand, exaggerated inflammation after myocardial infarction can also have detrimental consequences, leading to larger infarcts, progression of adverse cardiac remodeling and the development of heart failure. Modulation of the immune response following myocardial infarction thus may be an intriguing target for improving myocardial recovery. As a consequence, modulation of inflammation in cardiovascular disease demands thorough understanding of the underlying pathophysiology. Our group is especially interested in cross talk and signaling cascades that trigger leukocyte recruitment to the injured myocardium after myocardial infarction. Besides local recruitment from the blood, leukocyte supply via increased leukocyte production in the bone marrow is of mayor relevance for post-ischemic myocardial inflammation, but little is known about the pathways that carry the signals for increased leukocyte demands from the site of injury to the hematopoietic system. How do danger signals get into contact with the hematopoietic niche? How do hematopoietic stem and progenitor cells receive information about leukocyte demand? Do danger signals directly interact with hematopoietic stem cells or do they mediate cross talk to supply cells in the hematopoietic niche, e.g. endothelial cells? Exaggerated or prolonged leukocyte supply has been shown to increase adverse cardiac remodeling and increase the risk for secondary cardiovascular events due to atherosclerotic plaque inflammation.
Figure II Myocardial infarction triggers the release of a burst of pro-inflammatory danger signals from the infarct tissue into the blood. These cytokines may be involved in local immunoregulatory functions and the recruitment and transmigration of inflammatory leukocytes from the blood into the injured myocardium. Next to involvement in local recruitment, danger signals travel in the blood or via blood-independent routes for long-distance signaling. Leukocyte production by the hematopoietic system is responsible for constant supply of inflammatory cells. Increased hematopoiesis in the bone marrow and via extramedullary hematopoiesis replenishes the exhausting blood pool. However, exaggerated or prolonged leukocyte supply has been shown to increase adverse cardiac remodeling and increase the risk for secondary cardiovascular events due to atherosclerotic plaque inflammation. Graphics adopted from Servier art.

Prof. Dr. med. Timo Heidt, MD
Group leader
E-Mail: timo.heidt@uniklinik-freiburg.de
Tel.: +49-761-270-70420
Fax: +49-761-270-70450

Technician
Carolin Wadle

Postdoc
Dr. med. Miriam Krimmer

Postdoc
Dr. med. Hana Seung

Postdoc
Christian Weber

MD student
Timon Bühler

PhD student
Diana Chiang Jurado

MD student
Claus Jülicher

MD student
Johannes Studier-Fischer

MD student
Julien Thielmann
2019
Psychiatric Presentation of Anti-NMDA Receptor Encephalitis.
Endres D, Rauer S, Kern W, Venhoff N, Maier SJ, Runge K, Süß P, Feige B, Nickel K, Heidt T, Domschke K, Egger K, Prüss H, Meyer PT, Tebartz van Elst L.
Front Neurol. 2019 Nov 5;10:1086. [PubMed]
Real-time magnetic resonance imaging - guided coronary intervention in a porcine model.
Heidt T, Reiss S, Krafft AJ, Özen AC, Lottner T, Hehrlein C, Galmbacher R, Kayser G, Hilgendorf I, Stachon P, Wolf D, Zirlik A, Düring K, Zehender M, Meckel S, von Elverfeldt D, Bode C, Bock M, Mühlen CVZ.
Sci Rep. 2019 Jun 17;9(1):8663. [PubMed]
Nationwide outcomes of aortic valve replacement for pure aortic regurgitation in Germany 2008-2015.
Stachon P, Kaier K, Heidt T, Bothe W, Zirlik A, Zehender M, Bode C, von Zur Mühlen C.
Catheter Cardiovasc Interv. 2019 Jun 4. doi: 10.1002/ccd.28361. [PubMed]
Risk-Adjusted Comparison of In-Hospital Outcomes of Transcatheter and Surgical Aortic Valve Replacement. Stachon P, Kaier K, Zirlik A, Bothe W, Heidt T, Zehender M, Bode C, von Zur Mühlen C.
J Am Heart Assoc. 2019 Apr 2;8(7):e011504. doi: 10.1161/JAHA.118.011504. [PubMed]
2018
Purinergic receptor Y2 (P2Y2)- dependent VCAM-1 expression promotes immune cell infiltration in metabolic syndrome.
Merz J, Albrecht P, von Garlen S, Ahmed I, Dimanski D, Wolf D, Hilgendorf I, Härdtner C, Grotius K, Willecke F, Heidt T, Bugger H, Hoppe N, Kintscher U, von Zur Mühlen C, Idzko M, Bode C, Zirlik A, Stachon P.
Basic Res Cardiol. 2018 Oct 18;113(6):45. [PubMed]
Risk factors and outcome of postoperative delirium after transcatheter aortic valve replacement.
Stachon P, Kaier K, Zirlik A, Reinöhl J, Heidt T, Bothe W, Hehn P, Zehender M, Bode C, von Zur Mühlen C. Clin Res Cardiol. 2018 Sep;107(9):756-762. [PubMed]
Coronary magnetic resonance imaging after routine implantation of bioresorbable vascular scaffolds allows non-invasive evaluation of vascular patency.
von Zur Mühlen C, Reiss S, Krafft AJ, Besch L, Menza M, Zehender M, Heidt T, Maier A, Pfannebecker T, Zirlik A, Reinöhl J, Stachon P, Hilgendorf I, Wolf D, Diehl P, Wengenmayer T, Ahrens I, Bode C, Bock M.
PLoS One. 2018 Jan 25;13(1):e0191413. [PubMed]
Inflammatory Pathways Regulated by Tumor Necrosis Receptor-Associated Factor 1 Protect From Metabolic Consequences in Diet-Induced Obesity.
Anto Michel N, Colberg C, Buscher K, Sommer B, Pramod AB, Ehinger E, Dufner B, Hoppe N, Pfeiffer K, Marchini T, Willecke F, Stachon P, Hilgendorf I, Heidt T, von Zur Muhlen C, von Elverfeldt D, Pfeifer D, Schüle R, Kintscher U, Brachs S, Ley K, Bode C, Zirlik A, Wolf D.
Circ Res. 2018 Mar 2;122(5):693-700. [PubMed]
2017
A molecular intravascular ultrasound contrast agent allows detection of activated platelets on the surface of symptomatic human plaques.
Maier A, Plaza-Heck P, Meixner F, Guenther F, Kaufmann BA, Kramer M, Heidt T, Zirlik A, Hilgendorf I, Reinöhl J, Stachon P, Bronsert P, Birkemeyer R, Neudorfer I, Peter K, Bode C, von Zur Mühlen C.
Atherosclerosis. 2017 Dec;267:68-77. doi: 10.1016/j.atherosclerosis.2017.10.029. Epub 2017 Oct 24. [PubMed]
Dual targeting improves capture of ultrasound microbubbles towards activated platelets but yields no additional benefit for imaging of arterial thrombosis.
Günther F, Heidt T, Kramer M, Khanicheh E, Klibanov AL, Geibel-Zehender A, Ferrante EA, Hilgendorf I, Wolf D, Zirlik A, Reinöhl J, Bode C, Peter K, Kaufmann BA, Mühlen CVZ.
Sci Rep. 2017 Nov 2;7(1):14898. doi: 10.1038/s41598-017-15080-7. [PubMed]
2016
Proliferation and Recruitment Contribute to Myocardial Macrophage Expansion in Chronic Heart Failure.
Sager HB, Hulsmans M, Lavine KJ, Moreira MB, Heidt T, Courties G, Sun Y, Iwamoto Y, Tricot B, Khan OF, Dahlman JE, Borodovsky A, Fitzgerald K, Anderson DG, Weissleder R, Libby P, Swirski FK, Nahrendorf M.
Circ Res. 2016 Sep 16;119(7):853-64. doi: 10.1161/CIRCRESAHA.116.309001. Epub 2016 Jul 21. [PubMed]
RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction.
Sager HB, Dutta P, Dahlman JE, Hulsmans M, Courties G, Sun Y, Heidt T, Vinegoni C, Borodovsky A, Fitzgerald K, Wojtkiewicz GR, Iwamoto Y, Tricot B, Khan OF, Kauffman KJ, Xing Y, Shaw TE, Libby P, Langer R, Weissleder R, Swirski FK, Anderson DG, Nahrendorf M.
Sci Transl Med. 2016 Jun 8;8(342):342ra80. doi: 10.1126/scitranslmed.aaf1435. [PubMed]
Molecular Imaging of Activated Platelets Allows the Detection of Pulmonary Embolism with Magnetic Resonance Imaging.
Heidt T, Ehrismann S, Hövener JB, Neudorfer I, Hilgendorf I, Reisert M, Hagemeyer CE, Zirlik A, Reinöhl J, Bode C, Peter K, von Elverfeldt D, von Zur Muhlen C.
Sci Rep. 2016 May 3;6:25044. doi: 10.1038/srep25044. [PubMed]
Atheroprotection through SYK inhibition fails in established disease when local macrophage proliferation dominates lesion progression.
Lindau A, Härdtner C, Hergeth SP, Blanz KD, Dufner B, Hoppe N, Anto-Michel N, Kornemann J, Zou J, Gerhardt LM, Heidt T, Willecke F, Geis S, Stachon P, Wolf D, Libby P, Swirski FK, Robbins CS, McPheat W, Hawley S, Braddock M, Gilsbach R, Hein L, von zur Mühlen C, Bode C, Zirlik A, Hilgendorf I.
Basic Res Cardiol. 2016 Mar;111(2):20. [PubMed]
2015
4D-cardiac CT and IVUS support stenting of left main compression due to an enlarged pulmonary artery.
Heidt T, Büttner HJ,Löffelhardt N, Minners J,Langer M,Neumann FJ, Bode C, Pache G.
EuroIntervention. 2015 Dec 22;11(8):e1. [PubMed]
Targeting Interleukin-1β Reduces Leukocyte Production After Acute Myocardial Infarction.
Sager HB, Heidt T, Hulsmans M, Dutta P, Courties G, Sebas M, Wojtkiewicz GR, Tricot B, Iwamoto Y, Sun Y, Weissleder R, Libby P, Swirski FK, Nahrendorf M.
Circulation. 2015 Nov 17;132(20):1880-90. [PubMed]
Preprocedural planning and implantation of a transcatheter aortic valve without the use of contrast agent. Reinöhl J, Pache G, Ahrens I, Heidt T, Psyrakis D, Latib A, De Marco F, Schröfel H, Beyersdorf F, Zehender M, Bode C, von Zur Mühlen C.
EuroIntervention. 2015 Jul 9;11(3). pii: 20150527-02. [PubMed]
Myocardial Infarction Activates CCR2(+) Hematopoietic Stem and Progenitor Cells.
Dutta P, Sager HB, Stengel KR, Naxerova K, Courties G, Saez B, Silberstein L, Heidt T, Sebas M, Sun Y, Wojtkiewicz G, Feruglio PF, King K, Baker JN, van der Laan AM, Borodovsky A, Fitzgerald K, Hulsmans M, Hoyer F, Iwamoto Y, Vinegoni C, Brown D, Di Carli M, Libby P, Hiebert SW, Scadden DT, Swirski FK, Weissleder R, Nahrendorf M.
Cell Stem Cell. 2015 May 7;16(5):477-87. [PubMed]
Magnetic resonance imaging of bioresorbable vascular scaffolds: potential approach for noninvasive evaluation of coronary patency.
Reiss S, Krafft AJ, Zehender M, Heidt T, Pfannebecker T, Bode C, Bock M, von Zur Muhlen C.
Circ Cardiovasc Interv. 2015 Apr;8(4). pii: e002388 [PubMed]
Ischemic stroke activates hematopoietic bone marrow stem cells.
Courties G, Herisson F, Sager HB, Heidt T, Ye Y, Wei Y, Sun Y, Severe N, Dutta P, Scharff J, Scadden DT, Weissleder R, Swirski FK, Moskowitz MA, Nahrendorf M.
Circ Res. 2015 Jan 30;116(3):407-17. [PubMed]
2014
Chronic variable stress activates hematopoietic stem cells.
Timo Heidt, Hendrik B. Sager, Gabriel Courties, Partha Dutta, Yoshiko Iwamoto, Alex Zaltsman, Constantin von zur Muhlen, Christoph Bode, Gregory L. Fricchione, John Denninger, Charles P. Lin, Claudio Vinegoni, Peter Libby, Filip K. Swirski, Ralph Weissleder, Matthias Nahrendorf.
Nature Medicine 2014 June 22. [PubMed]
Dual contrast molecular imaging allows noninvasive characterization of myocardial ischemia-reperfusion injury after coronary vessel occlusion in mice by MRI.
von Elverfeldt D, Maier A, Duerschmied D, Braig M, Idzko M, Neudorfer I, Menzer, M, Zirlik A, Heidt T, Mauler M, Bode C, Peter K, von zur Muhlen C.
Circulation 2014 Jun 20. [PubMed]
Molecular Imaging of Vascular Thrombosis
Timo Heidt, Felix Günther, Marvin Krohn-Grimberghe, Karlheinz Peter, Christoph Bode and Constantin von zur Muhlen.
Current Molecular Imaging, 2014, 3, 27-36. [ingentaconnect]
Differential Contribution of Monocytes to Heart Macrophages in Steady-State and
After Myocardial Infarction.
Heidt T, Courties G, Dutta P,Sager H, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, Swirski FK, Weissleder R, Nahrendorf M.
Circ Res. 2014 May 1. [PubMed]
In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing.
Courties G, Heidt T, Sebas M, Iwamoto Y, Jeon D, Truelove J, Tricot B, Wojtkiewicz G, Dutta P, Sager HB, Borodovsky A, Novobrantseva T, Klebanov B, Fitzgerald K, Anderson DG, Libby P, Swirski FK, Weissleder R, Nahrendorf M. J
Am Coll Cardiol. 2014 Apr 22;63(15):1556-66. [PubMed]
2013
Multimodal iron oxide nanoparticles for hybrid biomedical imaging.
Heidt T, Nahrendorf M.
NMR Biomed. 2013 Jul;26(7):756-65. [PubMed]
Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice.
Majmudar MD, Keliher EJ, Heidt T, Leuschner F, Truelove J, Sena BF, Gorbatov R, Iwamoto Y, Dutta P, Wojtkiewicz G, Courties G, Sebas M, Borodovsky A, Fitzgerald K,Nolte MW, Dickneite G, Chen JW, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M.
Circulation. 2013 May 21;127(20):2038-46. [PubMed]
2012
Myocardial infarction accelerates atherosclerosis.
Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M.
Nature. 2012 Jul 19;487(7407):325-9. [PubMed]
2011
Enzymatic single-chain antibody tagging: a universal approach to targeted molecular imaging and cell homing in cardiovascular disease.
Ta HT, Prabhu S, Leitner E, Jia F, von Elverfeldt D, Jackson KE, Heidt T, Nair AK, Pearce H, von Zur Muhlen C, Wang X, Peter K, Hagemeyer CE.
Circ Res. 2011 Aug 5;109(4):365-73. [PubMed]
Activated platelets in carotid artery thrombosis in mice can be selectively targeted with a radiolabeled single-chain antibody.
Heidt T, Deininger F, Peter K, Goldschmidt J, Pethe A, Hagemeyer CE, Neudorfer I, Zirlik A, Weber WA, Bode C, Meyer PT, Behe M, von Zur Mühlen C.
PLoS One. 2011 Mar 30;6(3):e18446. [PubMed]
2007
Cyclin D1 expression is induced by viral BARF1 and is overexpressed in EBV-associated gastric cancer. Thorsten Wiech, Elisabeth Nikolopoulos, Silke Lassman, Timo Heidt, Anja Schöpflin, Mario Sarbia, Martin Werner, Yuko Shimizu, Emna Sakka, Tadamasa Ooka, Axel zur Hausen
Virchows Archive. 2007 Jun;452(6):621-7. [PubMed]
- Prof. Dr. Michael Bock, University of Freiburg, Germany
- Prof. Dr. Christoph Bode, University of Freiburg, Germany
- PD. Dr. Buß, University of Heidelberg, Germany
- PD Daniel Dürschmied, University of Freiburg, Germany
- PD Dr. Dominik von Elverfeldt, University of Freiburg, Germany
- Prof. Dr. Jörg Haberstroh, University of Freiburg, Germany
- Prof. Dr. Georg Häcker, University of Freiburg, Germany
- Prof. Dr. Christoph Hehrlein, University of Freiburg, Germany
- Prof. Dr. Marco Idzko, University of Freiburg, Germany
- PD Dr. Beat Kaufmann, University of Basel, Switzerland
- Prof. Dr. Matthias Nahrendorf, CSB, MGH and Harvard Medical School, Boston, USA
- PD Dr. Gregor Pache, University of Freiburg, Germany
- Prof. Dr. Karlheinz Peter, Baker IDI Heart and Diabetes Center, Melbourne, Australia
- Dr. Hendrik Sager, Herzzentrum München, Germany
- Prof. Dr. Constantin von zur Mühlen, University of Freiburg, Germany
Awards and Funding
- 2019 DFG Sachbeihilfe: “Magnetresonanztomographie zur integrativen Darstellung sowie interventionellen Behandlung der koronaren Plaqueinflammation"
- 2019 DGK Rudi – Busse Young Investigator Award (Hana Seung)
- 2018 DGAF Posterpreis (Timo Heidt)
- 2017 DGK Herbsttagung - Posterpreis (Hana Seung)
- 2015 ESC Grant for Medical Research Innovation
- 2015 DFG Sachbeihilfe: “Aktivierte Thrombozyten als Regulatoren der Knochenmarksaktivierung und Hämatopoese nach dem Myokardinfarkt”
- 2014 Edith-von-Kaulla Forschungspreis der Universität Freiburg
- 2012-2014 DFG Forschungsstipendium für ein Postdoc am Center for Systems Biology, MGH and Harvard Medical School, Boston, USA