Metabolism and Inflammation Research Group
Head: O. GroßWe explore danger sensing and innate immune signalling in macrophages, investigating their intricate connections with metabolism, cell death, and cellular structures. Macrophages exhibit fascinating cross-talk related to cell death, as they respond to danger signals released during cellular demise and can engage various genetically defined cell death pathways, each with varying inflammatory potential, some of which are triggered as a consequence of innate immune signalling. We are especially interested in the sensing mechanism of sterile endogenous or environmental danger signals and in indirect danger sensing such as involving the effects of pathogens on host cell signalling and function. Metabolism and organelles emerge as information-bearing signalling entities in this context.
Our primary focus lies on two families of pattern recognition receptors: inflammasomes and ITAM-coupled receptors that both not only respond to pathogens, but also recognize sterile danger signals. Inflammasomes, nucleated by cytoplasmic receptors, control the unconventional release of interleukin-1 family cytokines and trigger a lytic form of cell death called pyroptosis via their constituent protease caspase-1. On the cell surface, ITAM-coupled receptors engage NF-κB transcription factors, leading to macrophage activation and the expression of proinflammatory cytokines, as well as providing an essential inflammasome priming signal.
Our investigations of the functional consequences of these mechanisms in the context of development, homeostasis, host defence, immune pathology, and cancer extend to various tissues, including the brain, heart, lung, and bone marrow. Ultimately, our research aims to contribute fundamental insights into the molecular mechanisms of innate immunity and inflammation. By identifying rational targets, we hope to steer immunity and develop control-of-function tools, particularly for cancer-related applications.
Personalia
| Prof. Dr. Olaf Groß | Principle Investigator | +49 761 270 63097 |
| Dr. Oliver Gorka | Postdoc/Lab Manager | +49 761 270 63095 |
| Dr. Christoph Koentges | Postdoc | +49 761 270 63114 |
| Dr. Emilia Neuwirt | Postdoc | +49 761 270 63138 |
| Dr. Svenja Wöhrle | Postdoc | +49 761 270 63141 |
| Clara Dufossez | PhD Student | +49 761 270 63141 |
| Larissa Fischer | PhD Student | +49 761 270 63141 |
| Nadja Ortolf | PhD Student | +49 761 270 63143 |
| Xipeng Wang | PhD Student | +49 761 270 63139 |
| Elena Puma | Cand. med. | +49 761 270 63144 |
| Nora Fischenich | Cand. med. | +49 761 270 63138 |
| Isabella Ingerl | Cand. med. | +49 761 270 63139 |
| Linn Schönewolff | Cand. med. | +49 761 270 63138 |
| Ahmet Hakan Görkay | M.Sc. Student | +49 761 270 63144 |
| Jennifer Ruf | Student Assistant | +49 761 270 63144 |
Research Topic
Mechanisms of imidazoquinole-mediated Inflammasome activation
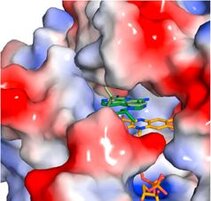
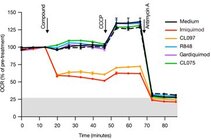
Imiquimod acts as a TLR7 ligand and has been licenced for treatment of viral infections and melanoma. Additionally, Imiquimod activates the NLRP3 inflammasome in myeloid cells and has the capacity to trigger apoptosis in tumour cells. We studied the mechanisms by which Imiquimod and other imidazoquinolines activate the inflammasome and found a K+ efflux-independent mode of action that involves the quinone oxidoreductases NQO2 and mitochondrial Complex I. Here, Imiquimod induces the production of reactive oxygen species (ROS) and leads to thiol oxidation which in turn activates NLRP3 via NEK7, a NIMA-related serine/threonine kinase. Our studies demonstrated a novel K+ efflux-independent mechanism for NLRP3 inflammasome activation and suggested clinically relevant targets of Imiquimod (Groß CJ et al. 2016, Immunity).
Figure: Crystal structure of NQO2 in complex with imiquimod. Surface representation of the active site of NQO2 in complex with imiquimod (Groß CJ et al. 2016, Immunity).

Prof. Dr. Olaf Groß
UNIVERSITÄTSKLINIKUM FREIBURG
Institut für Neuropathologie, IMITATE
Breisacher Straße 113
79106 Freiburg
Tel. +49 761 270 63097