Mx Proteins: Large GTPases Involved in Host Defense against RNA Viruses
In collaboration with:
- Dr. Oliver Daumke, MDC-Berlin: Structural analysis of Mx proteins
- Dr. Harmit S. Malik, Howard Hughes Medical Institute, Seattle: Evolution of primate Mx genes
- Dr. Marcel Müller and Dr. Christian Drosten, Virology, University Hospital, Bonn: Mx proteins from bats and rodents
- Dr. M. Schwemmle, Institute of Virology, Freiburg: Identification of Mx-associated viral and cellular factors
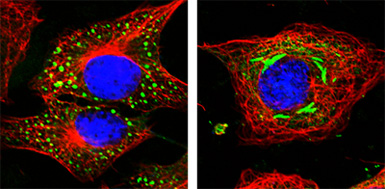
Human MxA GTPase interferes with intracellular transport of essential viral components.
Membrane-associated MxA blocks the transport of LaCrosse virus nucleocapsid protein to the Golgi compartment by sequestering the viral protein into large perinuclear aggregates.
(A) MxA (green) forms small dots in uninfected cells.
(B) After LaCrosse virus infection, MxA (green) redistributes together with the viral nucleoprotein into perinuclear complexes. The cytoskelleton is stained in red for microtubuli and the DNA is stained in blue.
Abstract
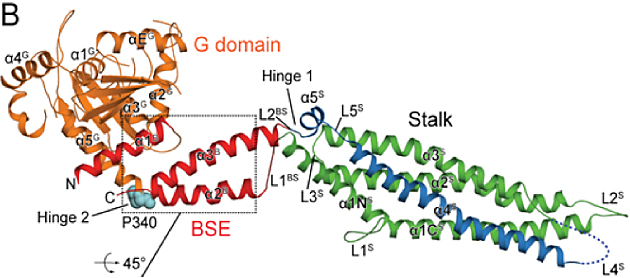
Mx proteins are key antiviral effector molecules which are induced by type I (alpha/beta) and type III (lambda) interferons and block an early step in the life cycle of specific human and animal viruses. Mice carrying a wild-type Mx1 gene are highly resistant to infection with even highly pathogenic influenza A viruses compared to Mx1-negative mice. Importantly, Mx proteins have an intrinsic, cell-autonomous antiviral activity. Available data indicate that Mx proteins work like pathogen-associated pattern-recognition (PAMP) receptors and oligomerize around viral nucleocapsids, thereby blocking their function. For example, the cytoplasmic MxA protein interacts with the ribonucleoprotein complex of the influenza-like Thogoto virus. This interaction leads to a block of transport of viral nucleocapsids into the nucleus, where viral transcription and replication takes place. Using strains of influenza viruses that differ in their sensitivity to Mx proteins, we showed that the viral nucleoprotein, the major component of the nucleocapsids of influenza viruses, constitutes the target of Mx action. In the case of bunyaviruses, like La Crosse virus, that have a cytoplasmic replication phase, association of MxA to the viral nucleocapsid protein leads to the sequestration of the viral protein into highly ordered perinuclear complexes and, as a consequence, to the inhibition of viral replication. Mx proteins belong to the dynamin family of large GTPases and consist of three functional domains, namely (i) the amino terminal “G domain” that binds and hydrolyses GTP, (ii) the “bundle signaling element” (BSE) and (iii) the “stalk” which mediates oligomerization and viral target recognition. We recently identified the flexible loop L4 of human MxA as an interface for viral target recognition and determinant of antiviral specificity. In future projects we want to characterize the physical interaction of Mx proteins with the viral target structure(s) and define the critical interaction domains. Furthermore, we will investigate the evolution of Mx proteins in rodents and bats and clarify their role in persistence of zoonotic viruses in these reservoirs. A long-term goal is to use the Mx - virus interaction platform as a blue-print for novel antiviral substances.
Selected References
- Mänz, B., Dornfeld, D., Götz, V., Zell, R., Zimmermann, P., Haller, O., Kochs, G., Schwemmle, M.
Pandemic influenza A viruses escape from restriction by human MxA through adaptive mutations in the nucleoprotein
Plos Path 9: e1003279 (2013) - Mitchell, P.S., Patzina, C., Emerman, M., Haller, O., Malik, H.S., Kochs, G.
Evolution-guided identification of antiviral specificity determinants in the broadly acting interferon-induced innate immunity factor MxA
Cell Host and Microbe 12: 1-7 (2012) - Zimmermann, P., Mänz, B., Haller, O., Schwemmle, M., Kochs, G.
The Viral Nucleoprotein Determines Mx Sensitivity of Influenza A Viruses
J Virol 85: 8133-8140 (2011) - Gao, S., Von der Malsburg, A., Dick, A., Faelber, K., Schröder, G.F., Haller, O., Kochs, G., Daumke, O.
Structure of Myxovirus Resistance Protein A Reveals Intra- and Intermolecular Domain Interactions Required for the Antiviral Function
Immunity 35: 514-525 (2011) - Von der Malsburg, A., Abutbul-Ionita, I., Haller, O., Kochs, G., Danino, D.
Stalk Domain of the Dynamin-like MxA GTPase Protein Mediates Membrane Binding and Liposome Tubulation via the Unstructured L4 Loop
J Biol Chem 286: 37858-37865 (2011) - Haller, O., Kochs, G.
Human MxA Protein: An Interferon-Induced Dynamin-Like GTPase with Broad Antiviral Activity
J Inteferon and Cytokine Research 31: 79-87 (2011) - Haller, O., Gao, S., von der Malsburg, A., Daumke, O., Kochs, G. (Review)
Dynamin-like MxA GTPase: Structural insights into Oligomerization and Implications for Antiviral Activity
J Biol Chem 285: 28419-28484 (2010) - Holzinger, D., Jorns, C., Stertz, S., Boisson-Dupuis, S., Thimme, R., Weidmann, M., Casanova, J.-L., Haller, O., Kochs, G.
Induction of MxA gene expression by influenza A virus requires type I or type III interferon signaling.
J Virol 81: 7776-7785 (2007)


Head:
Prof. Dr. med. Hartmut Hengel
hartmut.hengel@uniklinik-freiburg.de
| Secretary | Administration | Information desk |
|---|---|---|
Kristina Gendrisch Telefon: 0761 270-83480 Telefax: 0761 270-83479 | Gudrun Simpson Telefon: 0761 270-83711 Telefax: 0761 270-83703 | Jutta Schneeberger Telefon: 0761 270-83700 Telefax: 0761 270-83703 |