Elucidating the antiviral and immunomodulatory properties of IFN-λ
The recently discovered type III interferons (IFN), also known as IFN-λ, are part of the early innate immune response against viral infections. The IFN-λ system closely resembles the type I IFN (IFN-α/β) system in terms of expression after virus infection, as well as intracellular signaling and activation of antiviral host factors in susceptible cells. However, in contrast to type I IFN, which signals through a universally expressed cell surface receptor (IFNAR), IFN-λ uses a distinct receptor complex (IL28R) that is expressed on a limited range of cell types. Until recently, both the contribution of type III IFN to antiviral resistance, as well as the exact nature of IL28R-expressing cells in vivo remained elusive. Using knockout mice that lack functional receptors either for IFN-λ, IFN-α/β, or both, we identified a critical role of IFN-λ in epithelial antiviral host defence. For example, protection of the gut against rotavirus infection was severely compromised if the IFN-λ system was defective (see figure).
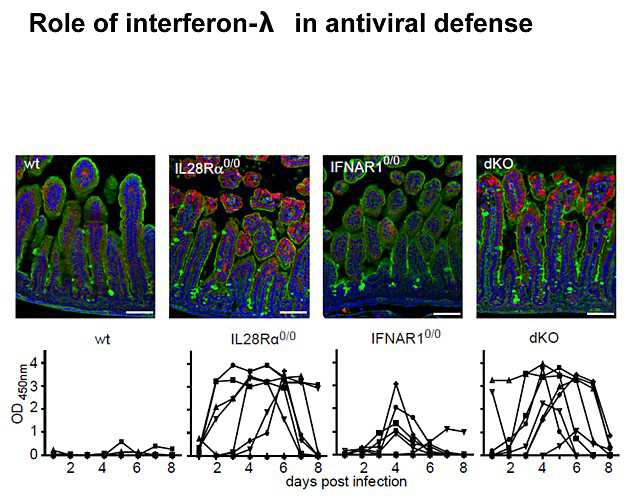
Enhanced rotavirus replication in mice lacking functional IFN-λ receptors.
(Top row) Suckling wild-type, IL28Rα0/0, IFNAR10/0 and IFNAR10/0IL28Rα0/0 mice were orally infected with murine rotavirus strain EDIM. Animals were sacrificed 4 d later, and immunostaining for rotavirus antigen (red) in thin sections of paraffin-embedded intestinal tissue was performed. Counterstaining was done with wheat germ agglutinin (WGA, green) and DAPI (blue).
(Bottom row) Virus shedding in feces of adult wild-type, IL28Rα0/0, IFNAR10/0 and IFNAR10/0IL28Rα0/0 (dKO) mice (n = 8 for each group) after oral infection with murine rotavirus strain EDIM. Each line represents the OD450nm values of one individual mouse during the observed time period. (Figure from Pott et al. 2011)
Enhanced rotavirus replication in mice lacking functional IFN-λ receptors. (Top row) Suckling wild-type, IL28Rα0/0, IFNAR10/0 and IFNAR10/0IL28Rα0/0 mice were orally infected with murine rotavirus strain EDIM. Animals were sacrificed 4 d later, and immunostaining for rotavirus antigen (red) in thin sections of paraffin-embedded intestinal tissue was performed. Counterstaining was done with wheat germ agglutinin (WGA, green) and DAPI (blue). (Bottom row) Virus shedding in feces of adult wild-type, IL28Rα0/0, IFNAR10/0 and IFNAR10/0IL28Rα0/0 (dKO) mice (n = 8 for each group) after oral infection with murine rotavirus strain EDIM. Each line represents the OD450nm values of one individual mouse during the observed time period. (Figure from Pott et al. 2011)
Open questions presently being investigated include:
a) Which cells and tissues express functional receptors for IFN-λ?
b) Which direct and indirect mechanisms of IFN-λ action are operative at mucosal sites?
Selected Publications from our group
- Mordstein M, Kochs G, Dumoutier L, Renauld J, Paludan SR, Klucher K, Staeheli P. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathogens 4/9: e1000151 (2008)
- Mordstein M, Neugebauer E, Ditt V, Jessen B, Rieger T, Falcone V, Sorgeloos F, Ehl S, Mayer D, Kochs G, Schwemmle M, Günther S, Drosten C, Michiels T, Staeheli P. Interferon-λ renders epithelial cells of respiratory and gastrointestinal tract resistant to viral infections. J. Virol. 84: 5670-5677 (2010)
- Pott J, Mahlakoiv T, Mordstein M, Duerr CU, Michiels T, Stockinger S, Staeheli P, Hornef MW. Interferon-λ determines the intestinal epithelial antiviral host defense. Proc. Natl. Acad. Sci. USA 108: 7944-7949 (2011)
- Bierne. H., Travier, L., Mahlakõiv, T., Tailleux, L., Subtil, A., Lebreton-Mansuy, A., Lebreton, A., Paliwal, A., Giquel, B., Staeheli, P., Lecuit, M., & P. Cossart. Activation of type III interferon genes by bacterial infections in epithelial cells and mouse placenta. PLoS One 7:e39080 (2012)
- Mahlakõiv, T., Ritz, D., Mordstein, DeDiego, M., Enjuanes, L., Müller, M., Drosten C., & P. Staeheli. Combined action of type I and type III IFN restricts initial replication of SARS-Coronavirus in the lung but fails to inhibit systemic virus spread. J. Gen. Virol. 93: 2601-2605 (2012)
- Crotta, S., Davidson, S., Mahlakoiv, T., Desmet, C.J., Buckwalter, M.R., Albert, M.L., Staeheli, P., & A. Wack. Type I and type III interferons drive redundant amplification loops to induce a transcriptional signature in influenza-infected airway epithelia. PLoS Pathogens 9/11: e1003773 (2013)
- Hermant, P., Demarez, C., Mahlakõiv, T., Staeheli, P., Meuleman, P., & T. Michiels. Human but not mouse hepatocytes respond to interferon-lambda in vivo. PLoS One 9/1: e87906 (2014)
- Mahlakõiv, T., Hernandez, P., Gronke, K., Diefenbach, A., & P. Staeheli. Leukocyte-derived IFN-α/β and epithelial IFN-λ constitute a compartmentalized mucosal defense system that restricts enteric virus infections. PLoS Pathogens 11/4: e1004782 (2015)
- Hernández, P., Mahlakõiv, T., Nguyen, N., Guendel, F., Ryffel, B., Hoelscher, C., Dumoutier, L., Renauld, J.-C., Staeheli, P., & A. Diefenbach. Interferon-λ and interleukin 22 act synergistically for the induction of interferon-stimulated genes and control of rotavirus infection. Nature Immunology 16: 698-707 (2015)
Collaboration
- Prof. Thomas Michiels, Université Catholique de Louvai, Brussels
- Prof. Mathias Hornef, Hannover Medical School, Hannover
- Prof. Andreas Diefenbach, University Medical Center Freiburg