MxGTPase: Mediator of influenza virus resistance in mice
Mice are frequently used as an animal model to study the pathogenesis of influenza A viruses However, standard laboratory mice do not possess a complete antiviral defense system, as they carry defective alleles of the Mx1 gene. Consequently, mice carrying a wild-type Mx1 gene (Mx1+/+) differ from standard laboratory mice (Mx1−/−) in being highly resistant to infection with influenza A virus. The Mx1 gene is under tight transcriptional control of alpha/beta interferon (IFN-α/β) and codes for a nuclear 72-kDa protein which represents a decisive antiviral factor that controls influenza virus infections in mice.
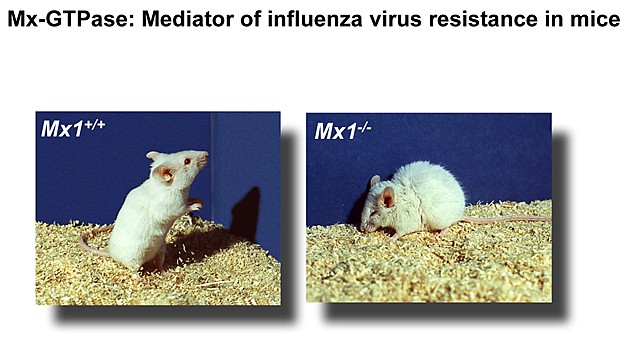
Health status of mice expressing (Mx1+/+) or lacking (Mx1-/-) functional alleles of the influenza virus resistance gene Mx1 at 4 days post infection with influenza A virus.
Open questions presently being investigated include:
a) Structural and functional analysis of Mx genes from wild mice
b) Viral resistance of transgenic mice expressing Mx gene of human origin
Selected Publications from our group
- Grimm D, Staeheli P, Hufbauer M, Koerner I, Martínez-Sobrido L, Solórzano A, García-Sastre A, Haller O, Kochs G. Replication fitness determines high virulence of influenza A virus in mice carrying functional Mx1 resistance gene. Proc. Natl. Acad. Sci. USA 104: 6806-6811 (2007).
- Tumpey T, Szretter KJ, van Hoeven N, Katz JM, Kochs G, Haller O, García-Sastre A, Staeheli P. The Mx1 gene protects mice against pandemic 1918 and highly lethal human H5N1 influenza viruses. J. Virol. 81: 10818-10821 (2007)
- Rolling, T., Koerner, I., Zimmermann, P., Holz, K., Haller, O., Staeheli, P. & G. Kochs. Adaptive mutations resulting in enhanced polymerase activity contribute to high virulence of influenza A virus in mice. J. Virol. 83: 6673-6680 (2009)
- Xiao, H., Killip, M.J., Staeheli, P., Randall, R.E., & D. Jackson. The human interferon-induced MxA protein inhibits early stages of influenza A virus infection by retaining the incoming viral genome in the cytoplasm. J. Virol. 87: 13053-13058 (2013)
- Verhelst, J., Spitaels, J., Nürnberger, C., De Vlieger, D., Ysenbaert, T., Staeheli, P., Fiers, W., & X. Saelens. Functional comparison of Mx1 from two different mouse species reveals the involvement of loop L4 in the antiviral activity against influenza A viruses. J. Virol. 89: 10879-10890 (2015)
- Pillai, P.S., Molony, R.D., Dong, H., Pang, I.K., Tal, M.C., Mohanty, S., Homer, R.J., Montgomery, R.R., Shaw, A.C., Staeheli, P., & A. Iwasaki. Mx1 reveals innate pathways to antiviral resistance and lethal influenza disease. Science 352: 463-466 (2016)
- Nürnberger, C., Zimmermann, V., Gerhardt, M., & P. Staeheli. Influenza virus susceptibility of wild-derived CAST/EiJ mice results from two amino acid changes in the MX1 restriction factor. J. Virol. 90: 10682-10692 (2016)
- Deeg, C.M., Hassan, E., Mutz, P., Rheinemann, L., Götz, V., Magar, L., Schilling, M., Kallfass, K., Nürnberger, C., Soubies, S., Kochs, G., Haller, O., Schwemmle M., & P. Staeheli. In vivo evasion of MxA by avian influenza viruses requires human signature in the viral nucleoprotein. J. Exp. Medicine 214: 1239-1248 (2017)
Collaboration
- Prof. Georg Kochs, Institute of Virology, University Medical Center Freiburg
- Prof. Martin Schwemmle, Institute of Virology, University Medical Center Freiburg
