Molecular and cellular characterization of aberrant myelination in focal cortical dysplasia
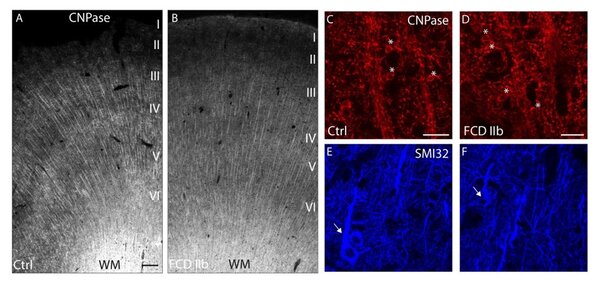
Myelination pattern and transcriptional regulation of myelin-associated genes in frontal lobe FCD IIb. Representative photomicrographs of CNPase-immunolabeled tissue sections from epileptic non-dysplastic (A) and FCD IIb (B) cases. In both groups myelinated fibers extend radially from the white matter up to layer II. Confocal images of CNPase/SMI32 double immunolabeling (C-F) demonstrate - myelinated fibers (red) and OL cell bodies (asterisks) and SMI32-positive pyramidal neurons (blue, arrows) in cortical layer V of control (C, E) and FCD IIb (D, F). Scale bars: A: 250 µm; C, D: 30 µm.
Funded by the German Research Foundation (DO 2542/1-1) and Müller-Fahnenberg-Stiftung
Funding to Dr. Catharina Donkels and Prof. Dr. Carola Haas
Project Description
Focal cortical dysplasias (FCDs) are local malformations of the human neocortex and a leading cause of medically intractable epilepsy. So far, neurosurgical resection of the epileptogenic focus is often the only therapeutic option to achieve seizure control. FCDs are a heterogeneous group of brain disorders which are characterized by local architectural disturbances of the neocortex, including dyslamination of neurons, the presence of dysmorphic neurons and/or balloon cells and a blurred gray-white matter boundary indicating abnormal white matter myelination. Little is known about myelination disturbances in the context of FCD so far and myelination abnormalities have been reported mostly for the white matter. As an intact myelin sheath is crucial for optimal conduction properties and the survival of axons, it is well conceivable that an imbalance of the myelin-axon interaction may contribute to FCD pathology. We have found by a whole transcriptome screening study that FCDs are associated with a myelination deficit in the gray matter. Specifically, transcripts encoding factors for oligodendrocyte differentiation and myelination are downregulated potentially due to diminished transcription factor/promoter binding capacities. Moreover, the densities of oligodendrocyte precursor cells (OPCs), myelinating oligodendrocytes (OLs) and of myelin fibers are drastically reduced in FCD. In addition, we found that OPCs have a reduced proliferation capacity in temporal lobe FCD IIa. Based on these findings we intend to perform a mapping of FCD type IIa and IIb in the temporal and frontal lobes to detect potential FCD type- and brain region-specific differences in myelination and oligodendrogenesis. We will use cortical tissue of FCD patients obtained after curative surgery to address (1) whether and how myelin sheaths are structurally altered, (2) whether transcriptional regulation of myelin genes is affected, (3) whether OL differentiation is compromised, and (4) whether the myelination capacity of OLs is reduced. We will address these questions by state-of the-art techniques including high-resolution confocal and electron microscopy, chromatin immunoprecipitation-sequencing and real time-qPCR. On the functional level, we will isolate and culture OPCs from resected human cortex, differentiate the OPCs into myelinating OLs and test their survival, differentiation and myelination capacity in vitro. Altogether, we expect to get important insights into the FCD type- and brain region-dependent dysregulation of myelination with respect to their myelination profile, transcriptional regulation during oligodendrogenesis, OL differentiation and myelination capacities. With this approach we hope to identify novel FCD type-specific features relevant for the development of new diagnostic tools and in the long run, with respect to medical therapy.