Neutrophils and NETs in Cardiovascular Disease
Neutrophils and NETs in Cardiovascular Disease
Principal Investigator: Dr. Lukas Heger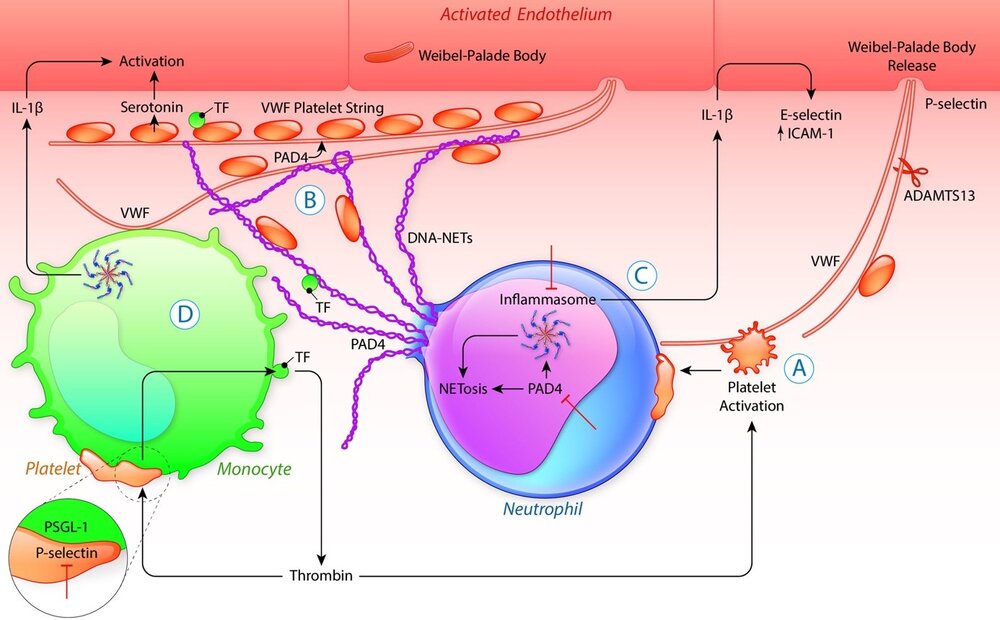
Once activated, platelets (A) form heterotypic, activating complexes with neutrophils and monocytes. The platelet-neutrophil interaction promotes extrusion of neutrophil extracellular traps (NETs; NETosis; B). NET production, in many cases, is preceded by neutrophil inflammasome assembly (C). Histones, as well as cytoplasmic and granular proteins entangled in NETs, have pleiotropic effects fueling pathological responses. Platelet-monocyte binding also induces inflammasome activation in monocytes (D). The inflammasome, through generation of active caspase 1, causes the release of the important vascular effector cytokine IL (interleukin)-1β. IL-1β in turn supports the endothelial expression of adhesion molecules such as E-selectin, propelling leukocyte recruitment. Activated platelets also stimulate TF (tissue factor) synthesis and its release in vesicles from monocytes (D). TF facilitates thrombin generation, fueling a systemic procoagulant state that with further platelet activation cumulates in the vicious cycle of thromboinflammation. Inhibition (┴) of PAD4, P-selectin/PSGL-1, inflammasome could potentially disrupt this process.
Our Mission:
To promote basic and applied discovery in the fields of neutrophil biology, heterocellular interactions and the thrombo-inflammatory response in cardiovascular disease and beyond.
What we are interested in:
To establish the activating interplay of neutrophil NLRP3 inflammasome and mechanisms that enable neutrophil extracellular traps release such as Protein Argini Deiminase 4 as targets for cardiovascular therapy in acute and chronic cardiovascular disease.

PI: Dr. med. Lukas A. Heger
Lukas is a MD and Clinical Scientist at the Department of Cardiology and Angiology, Faculty of Medicine, at University Hospital Freiburg in Germany. As a postdoctoral research fellow, funded by a grant from the German research foundation (DFG), he worked in the laboratory of Denisa Wagner at the Harvard Medical University investigating the role of peptidylarginine deiminase 4 (PAD4) and neutrophil extracellular traps (NETs) in adverse myocardial remodeling in chronic inflammation.

Lab manager: Daniela Stallmann

Clinician Scientist: Paula Liang

Clinician Scientist: Ines Derya Steenbuck
Working Group Cardiac Proteomics

MD candidate Jonathan Häßner
Jonathan is in his 9th semester of medical school and investigating the temporal dynamics of neutrophil NLRP3 expression and NET release following ST-segment elevation myocardial infarction and ischemia/reperfusion injury.

MD candidate Lara Schoenig
Lara is investigating the role of neutrophil preactivation and NET release in Transthyretin amyloid cardiomyopathy

Ali Emre Güler
MD-Student

Mitra Tajik
MD-Student

Anna Ohngemach
MD-Student
Current Projects:
- IRIS Study
- NETs in Amyloidose
- The platelet C5a-C5aR-axis and NET release
- Right Ventricular strain as predictive factor in transjugular intrahepatic portosystemic shunt (TIPS) outcome
- Heger, L. A., N. Schommer, S. Van Bruggen, C. E. Sheehy, W. Chan and D. D. Wagner (2024). "Neutrophil NLRP3 promotes cardiac injury following acute myocardial infarction through IL-1beta production, VWF release and NET deposition in the myocardium." Sci Rep 14(1): 14524.
- Heger, L. A., N. Schommer, S. Fukui, S. Van Bruggen, C. E. Sheehy, L. Chu, S. Rajagopal, D. Sivanandhan, B. Ewenstein and D. D. Wagner (2023). "Inhibition of protein arginine deiminase 4 prevents inflammation-mediated heart failure in arthritis." Life Sci Alliance 6(10).
- Wagner, D. D. and L. A. Heger (2022). "Thromboinflammation: From Atherosclerosis to COVID-19." Arterioscler Thromb Vasc Biol 42(9): 1103-1112.

