
PD Dr. Felicitas Bucher, MD, Dipl. Vwl.
Group Members
- Katharina Blatz, MD student
- Alban Hospach, MD
- Malte Jung, MD
- Jan Niklas Ness, Dipl.-Pharm., PhD Student
- Jessica Plura, Dipl.-Pharm, PhD student
- Melanie Schwämmle, MSc Mol Med, PhD Student
Former group Members
- Dr. Julian Rapp, MD
- Jakob Arnold, M.Sc.
- Sophie Krüger, technician
- Paula Liang, MD student
Collaborations
- Prof. Dr. M. Friedlander, The Scripps Research Institute, San Diego, USA
- Prof. Dr. O. Groß, Institute für Neuropathologie Freiburg
- Dr. L. Hannibal, Pädiatrisches Stoffwechselzentrum, Freiburg
- Prof. Dr. M. Hug, Klinikumsapotheke Freiburg
- Prof. Dr. I. Nazarenko, Tumor and Exosome Biology Group, Institut für Umweltmedizin Freiburg
- Prof. Dr. Schachtrup, Molecular Embryology, Institut für Anatomie und Zellbiologie Freiburg
- Prof. Yea, Daegu Gyeongbuk Institute of Science and Technology (DGIST), South Korea
Funding
- Berta-Ottenstein Program for Clinician Scientists
- Deutsche Forschungsgemeinschaft (DFG)
- Research Prize of the German Retina Society funded by the Dr. Werner Jackstädt Foundation for basic research in ophthalmology
- Else-Kröner-Fresenius-Stiftung
- Volker Homann Stiftung
- BMBF
- Förderpreis für Augenheilkunde (Bayer)
Retinal Angiogenesis, Makroglia, Stat3 Signaling
Research focus
Retinal vasoproliferative diseases represent common causes of blindness in the western world. Our research focusses on the pathophysiology of retinal vasoproliferative diseases, especially Stat3 signaling as well as the interaction of macroglial and vascular endothelial cells. In a translational approach we combine in vitro and in vivo models with clinical studies to understand the pathophysiology of retinal neovascular diseases.
Stat3 signaling in retinal angiogenesis
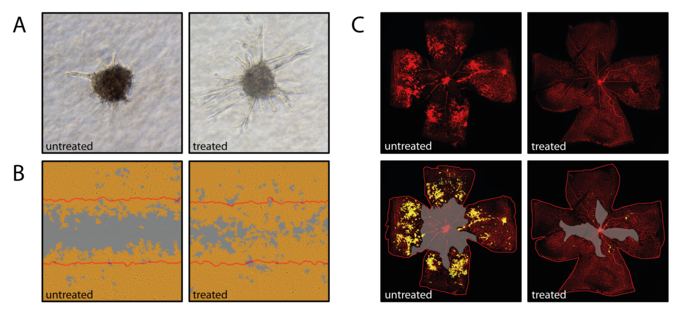
Interleukin 6 (IL6)-family cytokines and downstream Jak/Stat3 signaling have been associated with diabetic macular edema, proliferative diabetic retinopathy and age-related macular degeneration. While Stat3 signaling represents a promising therapeutic target in cancer-associated inflammation and angiogenesis, the contribution of IL6-induced Stat3 signaling to retinal neovascular diseases is still poorly understood. IL6-family cytokines seem to differ in their angiomodulatory function suggesting that the effect of IL6-family induced Jak/Stat3 signaling is tightly regulated and strongly context-dependent. We currently focus on characterizing the direct effect of Stat3 activation or knock-down on vascular endothelial cells using proliferation assays, wound healing assays (Figure 1B) and spheroid sprouting assays (Figure 1A). The mouse model of oxygen-induced retinopathy (Figure 1 C), an established rodent model for proliferative retinopathy, is used to assess the effect of IL6-family induced Stat3 signaling in vivo. A Cre/LoxP system with an endothelial specific Cre-line is used to assess retinal angiogenesis under loss of Stat3 signaling during retinal development and the vasoproliferative OIR model.
Macroglial cells
Astrocytes and Müller glial represent the macroglial cells of the retina. While astrocytes play an important role throughout the entire central nervous system, Müller glial cell are unique to the retina and span all its layers. Next to other important functions including metabolic support to neurons and ion/water transport, Müller cells contribute to the regulation of the blood retinal barrier as well as the angio-modulatory microenvironment. We use primary Müller cell culture systems to better understand their angiomodulatory secretome under standard and hypoxic cell culture conditions. We are further interested in their role in Stat3-mediated angiomodulation.
Macular Telangiectasia Type 2
Macular Telangiectasia Type 2 (MacTel) is are rare form of maculopathy that slowly deteriorates central vision and therefore strongly affects patients daily life. As one of three study centers for MacTel in Germany, we enroll patients for the MacTel Project Registry (NHOR) as well as the Phase 3 Renexus (NT-501) clinical trial (NTMT-03). Renexus is an intravitreal implant that continuously secretes ciliary neurotrophic factor (CNTF). CNTF belongs to one of the best studied neurotrophic factors. In a Phase 2 clinical trial, it showed great potential in slowing the disease progression in MacTel. However, the mechanism behind CNTF’s effect in the retina is not fully understood. We study the effect of CNTF on vascular endothelial cells and Müller cells in vitro as well as in vivo. Furthermore, in collaboration with PD Dr. Heinrich we are developing alternative measures to better characterize and assess visual function loss in MacTel patients since visual acuity measurements alone often do not adequately reflect and measure functional limitations in a patient's daily life.
Extracellular Vesicles (EVs)
Extrazelluläre Vesikel (EVs) haben sich als Diagnose- und Prognose-Marker innerhalb der letzten Jahre aufgetan. EVs sind Nanometer-große Strukturen, die von einer Lipiddoppelschicht umgeben sind und Proteine, Lipide, DNA oder RNA transportieren können. Sie tragen als Kommunikationsplattform wesentlich zu zellulären Prozessen bei. Daher können EVs in allen Körperflüssigkeiten wie z.B. Blut gefunden werden. Veränderungen der EV-Konzentration oder derer molekularen Zusammensetzung können auf das Vorliegen oder Fortschreiten einer Erkrankung hinweisen. In unserern Forschungsprojekten untersuchen wir das EV-Profil in Glaskörper- und Blutproben von Patienten mit retinalen Gefäßerkrankungen sowie in Ansätzen primärer retinaler Zellkulturen.
Publications
ORCID ID Felicitas Bucher https://orcid.org/0000-0003-0081-1420

