Molecular Genetics Group
Head: PD Dr. K.P. KnobelochOur group aims to understand the molecular and biological function of distinct components of the Ubiquitin- and Ubiquitin like systems within the context of the whole organism. Therefore we generate and analyze conditional Knockout and Knock-In models with a special focus on the ISG15 modification system and Ubiquitin specific proteases.
Research Topics
Ubiquitin specific proteases (USPs)
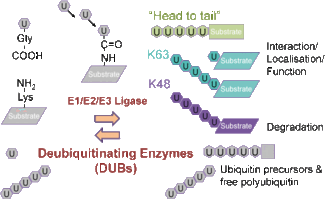
In analogy to phosphatases in protein phosphorylation ubiquitination of proteins is counteracted by the activity of Ubiquitin deconjugating enzymes (DUBs). Although much progress has been made in characterizing enzymes that link ubiquitin to proteins, the understanding of deubiquitinating enzymes is just beginning to evolve. The human genome contains more than 80 different DUBs, most of them belonging to the family of Ubiquitin specific proteases (USPs).
DUBs negatively regulate proteolysis or other signaling functions of ubiquitination such as internalization or altered protein function by removing the ubiquitin chain from the target. The Ubiquitin isopeptidase UBPY (USP8) represents a particular interesting member of deubiquitinating enzymes as the molecule is growth regulated and contains a structural motif for SH3 domain binding. Using conditional mutagenesis we generated mice that allow the time and cell specific inactivation of UBPY in the context of the whole organism. We were able to show that lack of UBPy results in embryonic lethality, whereas its induced inactivation in adults causes fatal liver failure. The defect is accompanied by a strong reduction or absence of several growth factor receptor tyrosine kinases (RTKs) like epidermal growth factor receptor (EGFR), hepatocyte growth factor receptor (c-met) and ERBB3. Consequently embryonic fibroblasts undergo growth arrest upon induced deletion of UBPy. UBPy deficient cells exhibit aberrantly enlarged early endosomes colocalizing with enhanced ubiquitination and have reduced levels of HRS and STAM2. Collectively our results demonstrate that UBPy is essential for receptor tyrosine kinase stability and to maintain proper endosomal transport in vivo. Currently these mice are used to elucidate the function of UBPY specifically in the brain and diverse subsets of immune relevant cell types.
Interferon stimulated gene 15 (ISG15)
Analogous to ubiquitin also other proteins with structural similarity- so called Ubiquitin like proteins (UBL) - can be covalently attached to target proteins and modify their function. Examples are SUMO, NEDD8, FAT10, and Interferon-stimulated gene 15 (ISG15/UCRP).
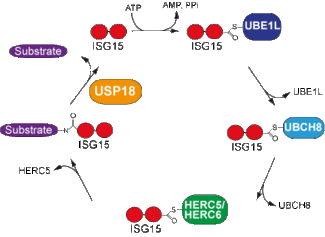
ISG15 was the first UBL described and represents one of the genes most strongly upregulated upon IFN induction. ISG15 is conjugated to a wide variety of target proteins and “Isgylation” is mediated by the activity of E1, E2 and E3 ligases. The conjugation represents a reversible process and ubiquitin specific protease 18 (USP18/UBP43) - which was originally described as an ubiquitin deconjugating enzyme - was reported to be an ISG15 specific isopeptidase. Using knockout animals generated in our lab, we investigate the function of the ISG15 modification system. We were able to show that while ISG15 is dispensable for antiviral activity against VSV and LCMV it serves as a critical molecule in the defence against Influenza and Herpes infections. The functional role of the interplay between ISG modification and the ubiquitin system on the ligase and deconjugation level is currently under investigation.

PD Dr. Klaus-Peter Knobeloch
University Clinic Freiburg
Institute of Neuropathology
Breisacher Straße 113
79106 Freiburg
Tel.: +49 761 270-63104
Fax: +49 761 270-50500
klaus-peter.knobeloch@uniklinik-freiburg.de