Cardiac Computational Biophysics
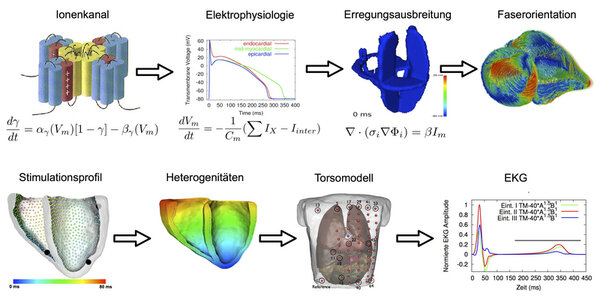
The "Cardiac Computational Biophysics" Group at the IEKM is working on the quantitative numerical description of the anatomy of the heart as well as the electrical and mechanical processes in the heart. This modeling refers to various scales of the description of the function of an ion channel protein to the realistic simulation of the ECG. One focus is on a better understanding of the physiological processes. Data of measurements of the other fields of the IEKM or other partners are integrated into the model, on the one hand to better understand the physiology and on the other hand, to complement measurements and their quantification. Using computational modelling also processes that have been measured in the animals are virtually transferred to humans to establish hypotheses about their impact. The simulation software is not only describing in detail the electric process including surface potentials but also the mechanical and the interaction of the two. This allows to quantify the consequences of the process on the ECG as well as on the deformation and the ejection fraction. In addition to the physiological processes, pathological conditions are reconstructed. Until now, the focus was lying on genetic defects, remodeling due to atrial fibrillation, ventricular ischemia, influence of fibrosis and myofibroblasts as well as the influence of drugs on the electrophysiology.
Example 1: Effect of Long QT syndrome (LQTS) on the mechanical deformation in rabbit
In this project, measured physiologic heterogeneities are integrated into the model of a rabbit. The LQTS is integrated by blocking the hERG ion channel. Firstly, the differences in electrophysiological processes including the T-wave are examined. Secondly, the impact on the mechanical deformation are described and compared with measured MRI data. The project aims to support a LQTS diagnosis by MRI.
Example 2: In silico quantification of the interaction between myocytes and myofibroblasts
Using computational modelling, the significance of the interaction of myocytes and myofibroblasts is examined. Based on measured data, the effects of the number of myofibroblasts and their coupling strength on the cellular electrophysiology and conduction are quantified. The project aims to better understand proarrhythmic effects of myofibroblasts.
- Giardini F, Olianti C, Marchal GA, Campos F, Romanelli V, Steyer J, Madl J, Piersanti R, Arecchi G, Vanaja IP, Biasci V, Rog-Zielinska EA, Nesi G, Loew LM, Cerbai E, Chelko SP, Regazzoni F, Loewe A, Bishop MJ, Mongillo M, Kohl P, Zaglia T, Zgierski-Johnston CM, Sacconi L. Correlative imaging integrates electrophysiology with three-dimensional murine heart reconstruction to reveal electrical coupling between cell types. Nat Cardiovasc Res 2025/in press
- Wülfers EM, Moss R, Lehrmann H, Arentz T, Westermann D, Seemann G, Odening KE, Steinfurt J. Whole-heart computational modelling provides further mechanistic insights into ST-elevation in Brugada syndrome. Int J Cardiol Heart Vasc. 2024/51:101373.
- Chleilat E, Walz TP, Quinn TA, Kohl P, Zgierski-Johnston CM. Perivascular excitation tunnelling as a novel mechanism of cardiac reperfusion arrhythmias. bioRxiv 2023
- Ohnemus, S., Vierock, J. & Schneider-Warme, F. Optogenetics meets physiology. Pflugers Arch - Eur J Physiol 2023/475:1369
- Ludwicki, K., Riebel, L.L., Ohnemus, S., Westby, F.M.E., Forsch, N., Balaban, G. An Automated Cardiac Constitutive Modelling Framework with Evolutionary Strain Energy Functions. In: McCabe, K.J. (eds) Computational Physiology. Simula SpringerBriefs on Computing/2023, vol 13.
- Winkelmann B, Zgierski-Johnston CM, Wülfers EM, Timmer J, Seemann G. Computational Mechanistic Investigation of Chronotropic Effects on Murine Sinus Node Cells. 2018 Computing in Cardiology Conference (CinC)
Team

Callum Zgierski-Johnston, PhD
Interim head of section

E-Mail: eike.wuelfers@uniklinik-freiburg.de
IT requests: iekm.itsupport-ticket@uniklinik-freiburg.de