PASADENA, PHIP, hydrogenative hyperpolarization
The use of the spin order of parahydrogen to enhance the available MR signal was devised in the 1980s. In contrast to SABRE, the para-spin order is made available to the molecule by a homogeneous hydrogenation reaction. By means of a subsequent r.f. sequence, the para-order may be transformed to high polarization on a third nucleus (Fig. 1, see spin order transfer).
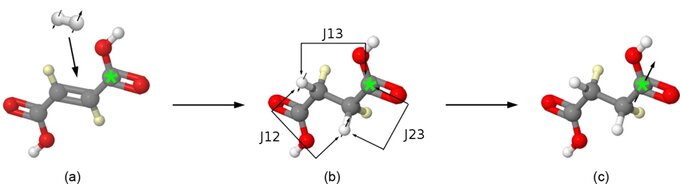
Figure 1: Scheme of the hyperpolarization process for [1-13C 2,3-2H] succinate. (a) Molecular parahydrogen is added to [1-13C 2,3-2H] fumarate by catalytic hydrogenation. (b) A pulse sequence is applied transferring the spin order to large, non-equilibrium polarization on the labelled carbon site (c)(*: 13C).
The following is a modified excerpt from Chekmenev et al, JACS, 2008:
One of the major shortcomings of NMR is poor sensitivity, which limits imaging of molecular concentrations in vivo to the millimolar level. Both DNP (dynamic nuclear polarization)1 and PASADENA (parahydrogen and synthesis allow dramatically enhanced nuclear alignment)(2, 3) have recently (4–11) been demonstrated to reach spin polarization of order unity on 13C sites with spin-lattice relaxation time T1 in the range of tens of seconds. This is a signal enhancement by a factor of ~100 000 on currently utilized MRI scanners. Exploring the potential for fast in vivo 13C imaging and spectroscopy is a principal motivation in these studies. While DNP was recently successfully applied to several metabolically relevant compounds including 1-13Cpyruvic acid(9,11), PASADENA at comparable polarization was demonstrated only with the ether 2-hydroxyethyl-propionate, which has no metabolic relevance.
Recently, the use of disodium 1-13C-acetylenedicarboxylate (ADC) as a precursor to hyperpolarized1-13C-succinate5 via the molecular addition of parahydrogen was demonstrated. However, that chemical approach suffered from
(i) a short spin-lattice relaxation time T1 of 6 s,
(ii) toxicity of the precursor ADC and intermediate maleate, which is relevant if hydrogenation is incomplete, and
(iii) polarization of only several percent (5).
The system described by (Chekmenev et al. 2008) overcomes these shortcomings.
We (Chekmenev et al. 2008) used 1-13C-fumaric acid-d2 as the unsaturated PASADENA precursor for the molecular addition of dihydrogen (Figure 1). In order to break the magnetic equivalence and simplify the spin dynamics, the 13C label is confined to only one carboxyl site (C1) in this otherwise symmetric molecule. The choice of an unprotonated carbon maximizes T1 for the hyperpolarized product species. Deuteration of the precursor at the positions 2 and 3 in the present approach (Figure 1) enables the increase of the crucial product T1 from 6 s (no deuteration) to 27 s (pH 3 and pH 7). A further increase to 39 s (pH = 3) and 56 s (pH = 7) was observed when the solvent was D2O.
After molecular addition of parahydrogen, an efficient transfer of spin order from the nascent protons to a third spin (2,7,8) requires precise information about the J-couplings among the three nuclei of succinate highlighted in red in Figure 1. A strong dependence of the couplings on pH has been reported (12) due to the changing probabilities of the interconverting neutral, anion, and dianion forms. We were motivated to revisit this problem with 1H coupled 13C spectroscopy of natural abundance succinic acid, since spectra needed to extract the couplings and dephasing of the three spins of interest here were not reported. The fits of 13C multiplet patterns of C1 and C2 carbons provide sufficient spectroscopic information for elucidation of these couplings.
Modified from "PASADENA Hyperpolarization of Succinic Acid for MRI", JACS, 2008
References
1. Abragam A, Goldman M. Rep Prog Phys 1978;41:395–467.
2. Bowers CR, Weitekamp DP. Phys Rev Lett 1986;57:2645–2648. [PubMed: 10033824]
3. Bowers CR, Weitekamp DP. J Am Chem Soc 1987;109:5541–5542.
4. Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Proc Natl Acad Sci 2003;100:10158–10163. [PubMed: 12930897]
5. Bhattacharya P, Chekmenev EY, Perman WH, Harris KC, Lin AP, Norton VA, Tan CT, Ross BD, Weitekamp DP. J Magn Reson 2007;186:108–113.
6. Bhattacharya P, Harris K, Lin AP, Mansson M, Norton VA, Perman WH, Weitekamp DP, Ross BD. Magn Reson Mat Phys Biol Med 2005;18:245–256.
7. Goldman M, Johannesson H, Axelsson O, Karlsson M. J Magn Reson Imaging 2005;23:153–157.
8. Goldman M, Johannesson H, Axelsson O, Karlsson M. C R Chimie 2006;9:357–363.
9. Golman K, Petersson JS. Acad Radiol 2006;13:932–942. [PubMed: 16843845]
10. Johannesson H, Axelsson O, Karlsson M. C R Physique 2004;5:315–324.
11. Kohler SJ, Yen Y, Wolber J, in’t Zandt R, Gram A, Ellner F, Thaning M, Chen A, Albers M, Bok R, Tropp J, Nelson S, Vigneron D, Kurhanewicz J, Hurd R. Proc Int Soc Magn Reson Med 2006;14:128.
12. Lit ES, Mallon FK, Tsai HY, Roberts JD. J Am Chem Soc 1993;115:9563–9567.<p/&
Dr. Andreas B. Schmidt
Head of Hyperpolarization
Tel.: +49 761 270-93880
E-Mail: andreas.schmidt@uniklinik-freiburg.de
University Medical Center Freiburg
Dept. of Radiology · Medical Physics
Killianstr. 5a
79106 Freiburg

