Duque Laboratory
Acute leukemias and allogeneic stem cell transplantationScientific focus
The work of our group is focused on the study of molecular pathomechanisms in acute leukemias and on translational studies in allogeneic hematopoietic cell transplantation.
Although the treatment of the acute leukemias has been improved during the last decades, there are still many patients not surviving the disease being relapse and chemotherapy-related complications the main causes of death. Most of survivors are young and therefore very susceptible to the negative long-term effects of chemo- and radiotherapy. In contrast, older patients or patients with comorbidities did not qualify for current intensified therapy regimens being treated with a palliative intention.
We aim to identify novel molecular targets and mechanisms of resistance to small molecules and chemotherapy using novel engineered leukemia mouse models as well as unbiased approaches as shRNA library screen, chromatin immunoprecipitation sequencing and global transcriptome analysis. We employ defined genetic models as the chimeric fusion proteins E2A-PBX1 in acute lymphoblastic leukemias and AML1-ETO in acute myeloid leukemia. Our long-term goal is to develop novel and more rational therapeutic approaches to contribute to the improvement of survival and quality of life of patients
A second major research focus of my group is the identification of molecular markers and clinical parameters including microbiome composition to assess risk for GvHD and relapse after allogeneic hematopoietic cell transplantation (allo-HCT) and for non-relapse mortality after autologous hematopoietic cell transplantation (auto-HCT). Although allo-HCT and auto-HCT are effective curative treatment options for several neoplastic and non-neoplastic diseases, these procedures are not devoid of complications.
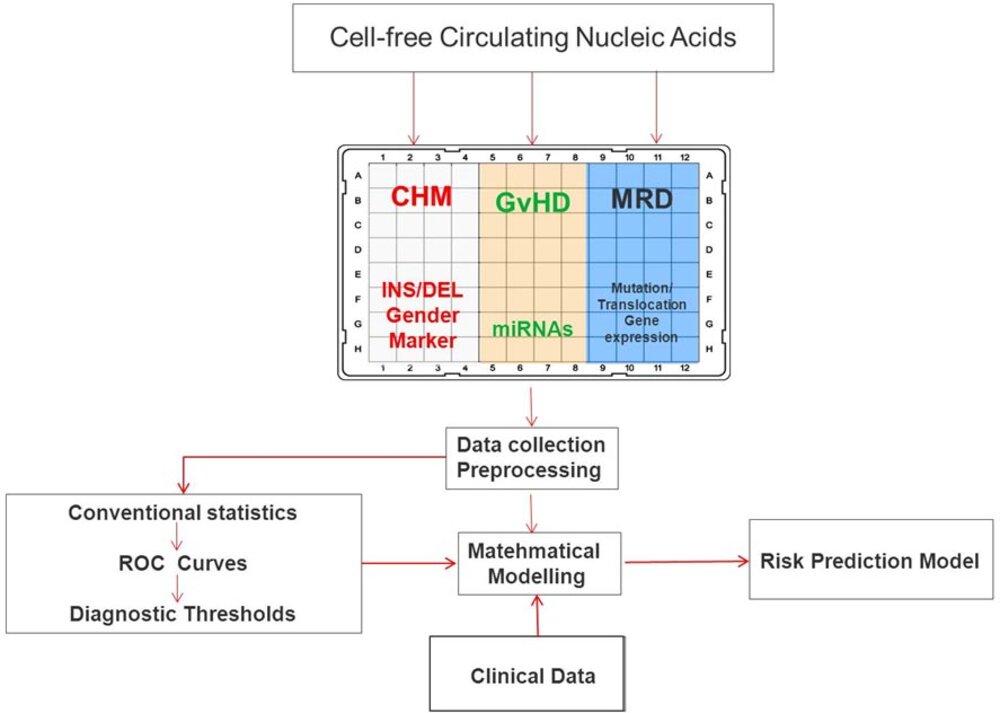
Relapse of the underlying disease and Graft-versus-Host Disease (GvHD) are frequent complications after allo-HCT, they contribute to morbidity and mortality and are the main cause of treatment failure. We aim to establish a non-invasive monitoring of circulating nucleic acids and to identify clinical parameters for early detection of relapse and/or GvHD in patients undergoing allo-HCT.
In addition, we are also investigating clinical factors, which contributes to non-relapse mortality in patients undergoing allo-HCT and auto-HCT, focusing especially on the pulmonary function. Our long term goal is to establish a disease risk predictive model, which will lead to preventive or anticipatory therapeutic interventions and improved outcomes of patients after allo-HCT and auto-HCT.
Team
Group leader
Prof. Dr. Jesús Duque-Afonso
Hematologist/Oncologist

Maria Yago Baenas
MD student
maria.yago.baenas@uniklinik-freiburg.de

Ariane Görgen
MD Student
ariane.goergen@uniklinik-freiburg.de

Heike Herzog
Medical Technical Assistant (MTA)
heike.herzog@uniklinik-freiburg.de

Sonja Jickeli
MD Student
sonja.jickeli@uniklinik-freiburg.de

Kristin Walther
MD Student
kristin.walther@uniklinik-freiburg.de

Thilo Thanscheidt
MD Student
thilo.thanscheidt@uniklinik-freiburg.de

Paraschiva Pitu
MD Student
paraschiva.pitu@uniklinik-freiburg.de

Mira Kusterer
MD Student
mira.kusterer@uniklinik-freiburg.de

Ernesto Olcina Aguado
MD Student
ernesto.olcina.aguado@uniklinik-freiburg.de

Radu-Florian Gherman
radu.florian.gherman@uniklinik-freiburg.de
Rebecca Berger
MD Student
Dr. rer. nat. Miguel Waterhouse
Dr. med. Gila Mostufi-Zadeh-Haghighi
M. Sc. Alexandra Brand
cand med. Miriam Eckert
Sophie Ewald, MD
Patricia Grüninger, MD
Johana Norona, PhD
Sandra Pennisi, PhD
Projects
Collaboration with Prof. Dr. M. Cleary and Prof. Dr. M. Bassik (Stanford University, CA, USA)
Collaboration with Prof. Dr. J. Finke (University of Freiburg, Germany).
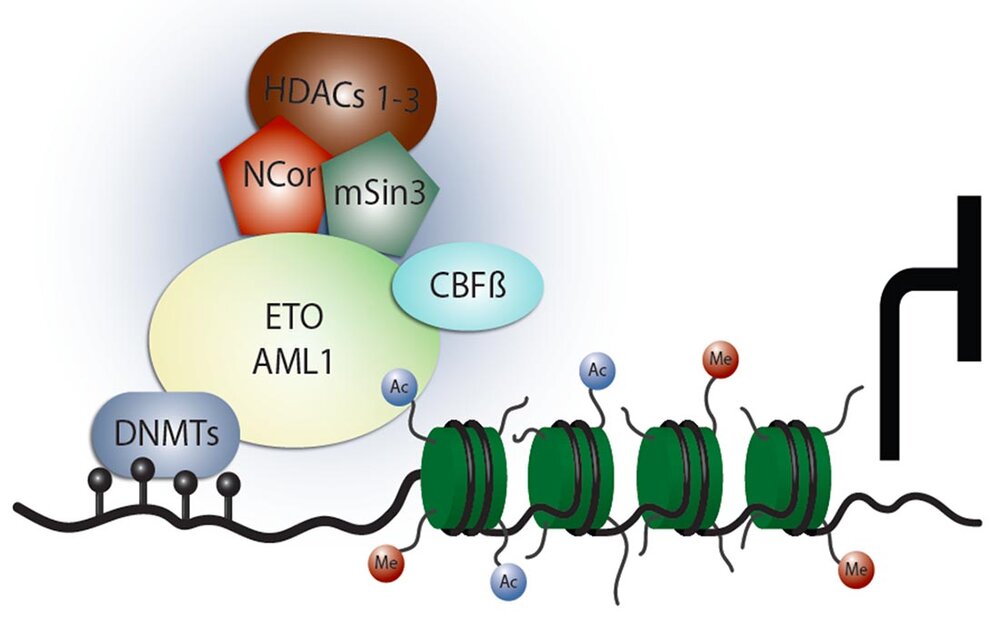
Collaboration with Prof. Dr. Michael Lübbert (University of Freiburg, Germany)
Further Information
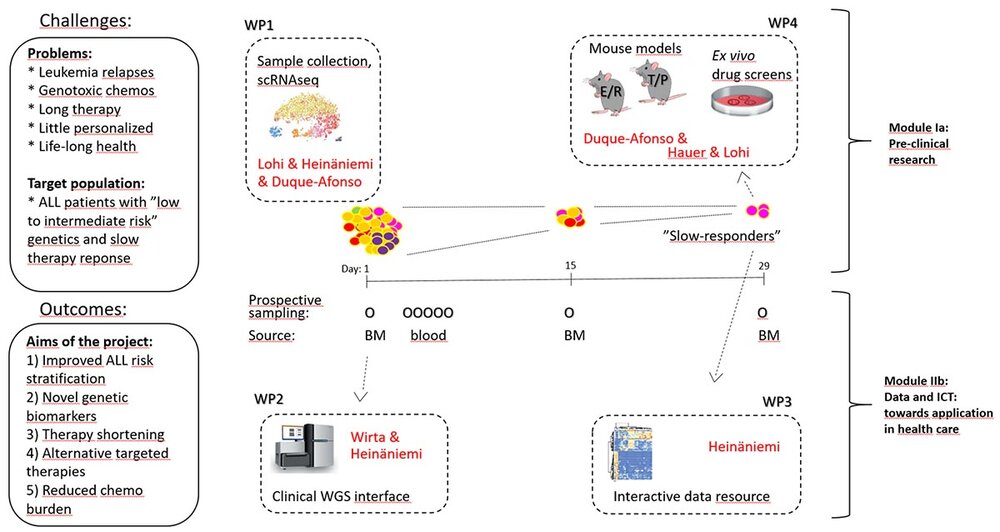
05/2025-04/2028 Research Grant from the EP PerMed co-founded by die European Commission for the project “Genomics- and biomarkers-based tools for personalized treatment to reduce chemotherapy burden in pediatric leukemia (GEPARD-2) ” (36 Monate) (01KU2515) .
07/2024 – 06/2027 Research grant from the Mertelsmann Foundation for the project “Development of artificial intelligence-driven tools based on cytomorphological analysis for diagnostic and outcome prediction of patients with acute hematological Diseases.
01/2020 - 12/2022 Research Grant from the German Research Foundation (DFG) for the project “Mechanisms of resistance to targeted therapy and chemotherapy in acute lymphoblastic leukemias”.
07/2019 - 06/2022 Research Grant from the ERA PerMed cofounded by the European Commission for the project “Genomics-based tools for personalized treatment to reduce chemotherapy burden in pediatric cancer (GEPARD)”.
01/2019 - 12/2020 Research Grant from Else Kröner-Fresenius-Stiftung for the project “Non-invasive monitoring of minimal residual disease and Graft-versus-Host Disease by circulating nucleic acids after allogeneic hematopoietic transplantation”.
01/2017 - 12/2017 Research Grant from the Research Committee (Forschungskommission) of the University of Freiburg for the project “Functional characterization of the TGFβ signalling pathway in acute lymphoblastic leukemia”.
Internal collaborations
- Dr. M. Kalweit, University of Freiburg Medical Center
- Prof. Dr. J. Finke, University of Freiburg Medical Center
- Prof. Dr. M. Lübbert, University of Freiburg Medical Center
- Prof. Dr. R. Zeiser, University of Freiburg Medical Center
- Prof. Dr. M. Engelhardt, University of Freiburg Medical Center
- PD Dr. R. Marks, University of Freiburg Medical Center
- Dr. C. Miething, University of Freiburg Medical Center
- PD Dr. R Sankowski, Neuropathology, University of Freiburg Medical Center
- Prof. Dr. B. Bengsch, Gastroenterology, University of Freiburg Medical Center
- Prof. Dr. O. Groß, Neuropathology, University of Freiburg Medical Center
- PD Dr. P. Bronsert, Pathology, University of Freiburg Medical Center
- Prof. Dr. T. Hartmann, Hematology/Oncology, University of Freiburg Medical Center
- Prof. Dr. O. Schilling, Pathology, University of Freiburg Medical Center
- PD Dr. F. Scherer, Dept. of Hematology/Oncology, University of Freiburg Medical Center
External collaborations
- Dr. F. Auer, TU Munich, Germany
- Prof. Dr. J. Nordlund, Uppsala University, Sweden.
- Prof. Dr. M. Cleary, Stanford University, CA , USA
- Prof. Dr. M. Bassik, Stanford University, CA, USA
- Prof. Dr. O. Lohi, University of Tampere and Tampere University Hospital, Finland
- Prof. Dr. M. Heinäniemi, University of Eastern Finland, Finland
- Prof. Dr. V. Wirta, Karolinska Institutet, Sweden
- Prof. Dr. J. Hauer, TU Munich, Germany
- Prof. Dr. A. Borkhardt, University of Düsseldorf, Germany
- Prof. Dr. G. Cario, University Medical Center Schleswig-Holstein (Kiel), Germany
- Prof. Dr. M. Schrappe, University Medical Center Schleswig-Holstein (Kiel), Germany
- Dr. Lennart Lenk, University of Kiel
- LABMaiTE, Freiburg.
Graphic design
- C. Duque-Afonso, PhD, University of Göttingen, Germany
Top publications
- Kusterer M, Lahnalampi M, Voutilainen M, Brand A, Pennisi S, Norona J, Gentile G, Herzog H, Greve G, Lübbert M, Sipola M, Kaartinen E, Sankowski R, Prinz M, Killmer S, Lago MS, Bengsch B, Cysar SR, Aumann K, Werner M, Duyster J, Lohi O, Heinäniemi M, Duque-Afonso J. (2025) Dynamic evolution of TCF3-PBX1 leukemias at the single-cell level under chemotherapy pressure. Hemasphere. Feb 3;9(2):e70071.
- Gentile G, Poggio T, Catalano A, Voutilainen M, Lahnalampi M, Andrade-Martinez M, Ma T, Sankowski R, Goncharenko L, Tholen S, Han K, Morgens DW, Prinz M, Lübbert M, Engel S, Hartmann TN, Cario G, Schrappe M, Lenk L, Stanulla M, Duyster J, Bronsert P, Bassik M, Cleary ML, Schilling O, Heinäniemi M, Duque-Afonso J. (2024) Development of combination therapies with BTK inhibitors and dasatinib to treat CNS-infiltrating E2A-PBX1+/preBCR+ ALL. Blood Adv. Apr 10:bloodadvances.2023011582.
- Duque-Afonso J, Veratti P, Rehman UU, Herzog H, Mitschke J, Greve G, Eble J, Berberich B, Thomas J, Pantic M, Waterhouse M, Gentile G, Heidenreich O, Miething C, Lübbert M. (2024) Identification of epigenetic modifiers essential for growth and survival of AML1/ETO-positive leukemia. Int J Cancer. Dec 1;155(11):2068-2079. doi: 10.1002/ijc.35134.
- Gherman RF, Ewald S, Ihorst G, Strüßmann T, Zeiser R, Wäsch R, Bertz H, Stolz D, Duyster J, Finke J, Marks R, Engelhardt M, Duque-Afonso J. (2023) Identification of clinical factors impacting outcome in patients undergoing autologous hematopoietic cell transplantation after BEAM and TEAM conditioning. Eur J Haematol.;. doi: 10.1111/ejh.14118
- Functional Characterization of Transforming Growth Factor- Signaling in Dasatinib Resistance and Pre-BCR+ Acute Lymphoblastic Leukemia.
Mostufi-Zadeh-Haghighi G, Veratti P, Zodel K, Greve G, Waterhouse M, Zeiser R, Cleary ML, Lübbert M and Duque-Afonso J.
Cancers 2023, 15(17), 4328; https://doi.org/10.3390/cancers15174328
- Monitoring of Measurable Residual Disease Using Circulating DNA after Allogeneic Hematopoietic Cell Transplantation.
Waterhouse M, Pennisi S, Pfeifer D, Scherer F, Zeiser R, Duyster J, Bertz H, Finke J, Duque-Afonso J.
Cancers (Basel). 2022 Jul 7;14(14):3307. https://doi.org/10.3390/cancers14143307.
- Functional characterization of the PI3K/AKT/MTOR signaling pathway for targeted therapy in B-precursor acute lymphoblastic leukemia.
Grüninger PK, Uhl F, Herzog H, Gentile G, Andrade-Martinez M, Schmidt T, Han K, Morgens DW, Bassik MC, Cleary ML, Gorka O, Zeiser R, Groß O, Duque-Afonso J.
Cancer Gene Ther. 2022 Nov;29(11):1751-1760. doi: 10.1038/s41417-022-00491-0
- Comparison of fludarabine-melphalan and fludarabine-treosulfan as conditioning prior to allogeneic hematopoietic cell transplantation-a registry study on behalf of the EBMT Acute Leukemia Working Party.
Duque-Afonso J, Finke J, Labopin M, Craddock C, Protheroe R, Kottaridis P, Tholouli E, Byrne JL, Orchard K, Salmenniemi U, Hilgendorf I, Hunter H, Nicholson E, Bloor A, Snowden JA, Verbeek M, Clark A, Savani BN, Spyridonidis A, Nagler A, Mohty M.
Bone Marrow Transplant. 2022 Aug;57(8):1269-1276. doi: 10.1038/s41409-022-01646-1
- The impact of pulmonary function in patients undergoing autologous stem cell transplantation.
Duque-Afonso J, Ewald S, Ihorst G, Waterhouse M, Struessmann T, Zeiser R, Wäsch R, Bertz H, Müller-Quernheim J, Duyster J, Finke J, Marks R, Engelhardt M.
Blood Adv. 2021 Nov 9;5(21):4327-4337. doi: 10.1182/bloodadvances.2021004863
- Colon and liver tissue damage detection using methylated SESN3 and PTK2B genes in circulating cell-free DNA in patients with acute graft-versus-host disease.
Waterhouse M, Pennisi S, Pfeifer D, Deuter M, von Bubnoff N, Scherer F, Strüssmann T, Wehr C, Duyster J, Bertz H, Finke J, Duque-Afonso J.
Bone Marrow Transplant. 2021 Feb;56(2):327-333. doi: 10.1038/s41409-020-01090-z
- Comparison of reduced-toxicity conditioning protocols using fludarabine, melphalan combined with thiotepa or carmustine in allogeneic hematopoietic cell transplantation.
Duque-Afonso J, Ihorst G, Waterhouse M, Zeiser R, Wäsch R, Bertz H, Yücel M, Köhler T, Müller-Quernheim J, Marks R, Finke J.
Bone Marrow Transplant. 2021 Jan; 56(1):110-120. doi: 10.1038/s41409-020-0986-2
- Droplet digital PCR for the simultaneous analysis of minimal residual disease and hematopoietic chimerism after allogeneic cell transplantation.
Waterhouse M, Pfeifer D, Duque-Afonso J, Follo M, Duyster J, Depner M, Bertz H, Finke J.
Clin Chem Lab Med. 2019 57(5):641-647. doi: 10.1515/cclm-2018-0827.
- Cell-free DNA characteristics and chimerism analysis in patients after allogeneic cell transplantation.
Duque-Afonso J, Waterhouse M, Pfeifer D, Follo M, Duyster J, Bertz H, Finke J.
Clin Biochem. 2018; 52:137-141. doi: 10.1016/j.clinbiochem.2017.11.015. - CBP Modulates Sensitivity to Dasatinib in Pre-BCR+ Acute Lymphoblastic Leukemia.
Duque-Afonso J, Lin CH, Han K, Morgens DW, Jeng EE, Weng Z, Jeong J, Wong SHK, Zhu L, Wei MC, Chae HD, Schrappe M, Cario G, Duyster J, Xiao X, Sakamoto KM, Bassik MC, Cleary ML.
Cancer Res. 2018; 78(22):6497-6508. doi: 10.1158/0008-5472.CAN-18-1703
Review articles and book chapters
- AML1/ETO and its function as a regulator of gene transcription via epigenetic mechanisms.
Rejeski K*, Duque-Afonso J*, Lübbert M.
Oncogene. 2021 Sep; 40(38):5665-5676. * Equally contribution
- The epigenetics of breast cancer - Opportunities for diagnostics, risk stratification and therapy.
Schröder R, Illert AL, Erbes T, Flotho C, Lübbert M, Duque-Afonso J.
Epigenetics. 2022 Jun; 17(6):612-624. - The AML salad bowl.
Duque-Afonso J, Cleary ML.
Cancer Cell. 2014 Mar 17; 25(3):265-7. doi: 10.1016/j.ccr.2014.03.002.
Complete List of Published Work in: