Translational Cancer Immunotherapy Lab
Decoding immune–cancer interactions with systems immunology, computational biology, and functional modeling.
About us
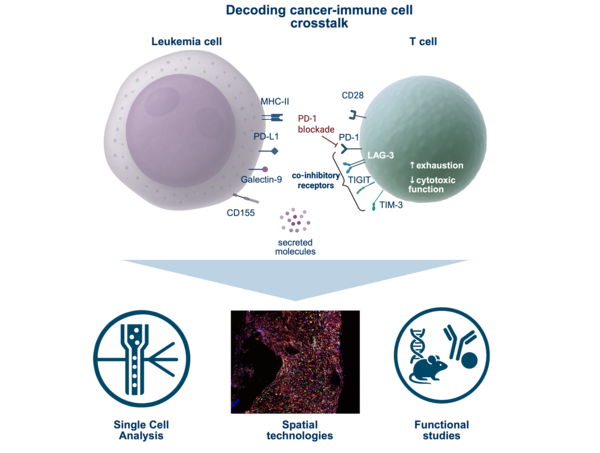
The Translational Cancer Immunotherapy Lab investigates how the tumor immune microenvironment drives cancer progression, therapy resistance, and relapse. We use systems immunology and computational biology approaches with single-cell and spatial technologies to map immune–cancer interactions at high resolution These insights are tested in advanced functional models, including genetically engineered mouse models. Through this integrative approach, we aim to translate mechanistic discoveries into innovative and personalized cancer immunotherapies. Through this integrative approach, we aim to translate mechanistic discoveries into innovative and personalized cancer immunotherapies.
Our research is driven by a dynamic, interdisciplinary team of clinician scientists, molecular biologists, and computational scientists. Together, we bring complementary expertise that bridges fundamental discovery and clinical application.
Decoding the Tumor Microenvironment
Our research begins with a central question: how do immune and cancer cells communicate within the tumor microenvironment to shape disease progression? We map these communication networks to uncover how immunosuppressive subsets, such as regulatory T cells (Wertheimer et al., Nature Immunology 2024), together with cytokine-driven signaling pathways, create niches that protect tumors and limit anti-tumor immunity. By integrating single-cell and spatial technologies with functional experiments in advanced models, we dissect these interactions at high resolution.
Building on these insights, we aim to turn communication networks into therapeutic entry points—identifying and targeting the mechanisms that underlie immune evasion in order to enhance the efficacy of cancer immunotherapies.
As a complementary line of research, we apply the same systems immunology methodologies to investigate toxicity in the context of cancer immunotherapy, mapping maladaptive communication patterns that drive adverse immune reactions. This dual perspective—on both tumor progression and treatment-associated toxicity—enables us to develop strategies that not only strengthen anti-tumor responses but also improve safety.
Relapse After Allogeneic Hematopoietic Cell Transplantation (allo-HCT)
A central focus of our research is relapse after allo-HCT, one of the major barriers to cure in hematologic malignancies. We apply systems-level profiling and functional models to dissect how residual cancer cells evade immune surveillance and resist graft-versus-leukemia effects. Our goal is to uncover mechanisms that can guide novel strategies for preventing and treating relapse.
Towards Personalized Immunotherapies
We integrate computational modeling and AI-driven analysis with translational studies to uncover determinants of therapy response, resistance, and relapse across multiple immunotherapy platforms, including CAR-T cells, immune checkpoint blockade, and allo-HCT. By identifying predictive biomarkers and targetable pathways, we aim to develop personalized immunotherapies that are both more effective and more durable.
TEAM
Head of Wertheimer Laboratory
Principal Investigator
Dr. Tobias Wertheimer
tobias.wertheimer@uniklinik-freiburg.de
Zentrum Translationale Zellforschung (ZTZ)
Breisacher Str. 115
D- 79106 Freiburg
Kornelia Fritsch
Lab Manager
Dr. Hosna Baniadam
Research associate
Utku Can Karaca
PhD student
Lina Schlenke
PhD student
lina.schlenke@uniklinik-freiburg.de
Annika Streit
Master’s thesis student
Sofoklis Trigkas-Chatziandreou
PhD student
Dr. Jakob Zillinger
Clinician scientist
Join Our Team

We are always open to initiative applications from PhD and MD students who are passionate about translational cancer immunology research. For PhD projects, we particularly welcome applicants with backgrounds in molecular biology, bioinformatics, computer science, or related fields. Our lab offers a highly interdisciplinary environment, combining cutting-edge single-cell technologies with functional experimental approaches. If you are excited to contribute to our projects—or to shape your own ideas within our research focus—we would be happy to hear from you.
1. The Department of Medicine I, Division of Hematology, Oncology and Stem Cell Transplantation, Center for Translational Cell Research (ZTZ), is looking for a
PhD student in Computational Biology / Bioinformatics / Systems Immunology (m/f/d)
The research project focuses on the cellular and molecular mechanisms of immune escape in acute myeloid leukemia (AML). Using state-of-the-art single-cell, multi-omics, and spatial analyses, the goal is to identify signaling pathways and transcriptional networks that govern the interaction between leukemia and immune cells.
Details
2. The Department of Medicine I, Division of Hematology, Oncology and Stem Cell Transplantation, Center for Translational Cell Research (ZTZ), is seeking, for the research group of Dr. Tobias Wertheimer, at the next possible date, a
PhD Student (m/f/d) for a basic and translational immunology research project
Topic: Immune Escape in Acute Myeloid Leukemia (AML)
Our goal is to understand the interactions between leukemia and immune cells that lead to immune evasion and relapse after allogeneic stem cell transplantation. To achieve this, we combine state-of-the-art experimental models with high-throughput single-cell and spatial technologies.
Details

- Florian Ingelfinger (Freiburg)
- Sascha Dietrich (Düsseldorf)
- Alexandra Nieters (Freiburg)
- Ian Frew (Freiburg)
- Wolfgang Huber, EMBL Heidelberg
- Nir Yosef, Weizmann Institute, Rehovot (Israel)
- Tilman Brummer, University of Freiburg
- Robert Zeiser, University Medical Center Freiburg
- Kimon Argyropoulos, Memorial Sloan Kettering Cancer Center, New York, USA
- Sonia Tugues, University of Zurich
- Burkhard Becher, University of Zurich
- Thorsten Zenz, University Hospital Zurich
- Guido Beldi, Inselspital Bern
- Dietmar Zaiss, University of Regensburg
- Kristina Maas-Bauer, University Medical Center Freiburg
- Natalie Köhler, University Medical Center Freiburg
1. Wertheimer, T., Zwicky, P., Rindlisbacher, L., Sparano, C., Vermeer, M., de Melo, B. M. S., Haftmann, C., Rückert, T., et al. IL-23 stabilizes an effector Treg cell program in the tumor microenvironment. Nat. Immunol. 25, 512–524 (2024) 10.1038/s41590-024-01755-7.
2. Nuñez, N. G., Schmid, J., Power, L., Alberti, C., Krishnarajah, S., Kreutmair, S., Unger, S., Blanco, S., …, Wertheimer, T. et al. High-dimensional analysis of 16 SARS-CoV-2 vaccine combinations reveals lymphocyte signatures correlating with immunogenicity.
Nat. Immunol. 24, 941–954 (2023) 10.1038/s41590-023-01499-w.
3. Ho, J. N. H. G., Schmidt, D., Lowinus, T., Ryoo, J., Dopfer, E.-P., Gonzalo Núñez, N., Costa-Pereira, S., Toffalori, C., Punta, M., Fetsch, V., Wertheimer, T., et al. Targeting MDM2 enhances antileukemia immunity after allogeneic transplantation via MHC-II and TRAIL-R1/2 upregulation. Blood 140, 1167–1181 (2022) 10.1182/blood.2022016082.
4. Wertheimer, T. & Tugues, S. IFN-λ: paving the road toward tissue protection in GVHD. Blood 138, 596–597 (2021) 10.1182/blood.2021012348.
5. Wertheimer, T., Velardi, E., Tsai, J., Cooper, K., Xiao, S., Kloss, C. C., Ottmüller, K. J., Mokhtari, Z., et al. Production of BMP4 by endothelial cells is crucial for endogenous thymic regeneration. Science Immunology. 3, eaal2736 (2018) 10.1126/sciimmunol.aal2736.
6. Velardi, E., Tsai, J. J., Radtke, S., Cooper, K., Argyropoulos, K. V., Jae-Hung, S., Young, L. F., Lazrak, A., …, Wertheimer, T., et al. Suppression of luteinizing hormone enhances HSC recovery after hematopoietic injury. Nat. Med. 24, 239–246 (2018) 10.1038/nm.4470.
2024 | John Hansen Research Grant (DKMS) |
| 2023 | Abstract Achievement Award (American Society of Hematology) |
| 2023 | Young Investigator Award, Annual Meeting of the German, Austrian and Swiss Societies for Hematology and Oncology |
| 2021 | Hans Jochem Kolb Award for Advances in Allogeneic Stem Cell |
| 2021-2023 | Walter-Benjamin Fellowship of the Deutsche Forschungsgemeinschaft |
| 2020 | ReForm A Fellowship for Young Investigators, University of Regensburg |
| 2019 | Regensburg Oncology Award (Comprehensive Cancer Center Regensburg) |
| 2018 | Jon J. van Rood Award (EBMT with E. Velardi and J. Tsai) |
| 2014 | Abstract Achievement Award (American Society of Hematology) |
| 2013-2014 | MD Fellowship, Boehringer Ingelheim Fonds |
| 2012-2017 | Scholar of the German National Academic Foundation (Studienstiftung des deutschen Volkes) |