Talvard-Balland Laboratory
About usOur laboratory is dedicated to investigating immune responses following allogeneic transplantation.
Our work focuses on dissecting immune escape mechanisms and enhancing anti-tumor immunity, while also investigating regulatory pathways that may help restrain graft-versus-host disease (GvHD).
We employ preclinical animal models and advanced imaging technologies to identify and develop innovative therapeutic strategies that improve patient outcomes.
Scientific focus
Focus 1: Deciphering immune cell-tumor cell interactions and improving immunotherapies
We investigate how hematological malignancies evade immune surveillance, with particular emphasis on the contribution of oncogenic signaling in tumor immune escape. We also investigate the complex interactions between immune cells and tumor cells, aiming to identify mechanisms to enhance anti-tumor responses and improve immunotherapies. We use advanced in vivo imaging capabilities for monitoring tumor progression, treatment response, and immune dynamics in real time and in longitudinal studies.
Focus 2: T-Cell Regulatory Subsets for Cell Therapy in GvHD
We study T-cell regulatory subsets to develop cell-based therapies to prevent or treat graft-versus-host disease (GvHD). By characterizing their function and mechanisms of immune regulation, we aim to harness these cells to improve transplant outcomes while maintaining anti-tumor immunity.
Team

Principal Investigator
Nana Talvard-Balland, Ph.D
nana.talvard-balland@uniklinik-freiburg.de
+49 761 270-71821
ORCID iD : 0000-0001-8590-6351
UniversitätsKlinikum Freiburg
Department of Hematology, Oncology and Stem Cell Transplantation (ZTZ)
Breisacher Straße 115 | 79106 Freiburg, Germany
Andrea Acerbis, visiting MD scientist, acerbis.andrea@hsr.it
Jule Ehmann, Master student (Biology M.Sc), jule_ehmann@icloud.com
Cooperations
- Karen O. Dixon (Basel, Switzerland)
- David Michonneau (Paris, France)
- Livius Penter (Berlin, Germany)
- Peter Van Galen (Boston, USA)
- Bruce Blazar (Minnesota, USA)
- Cathrin Wu (Boston, USA)
- Erica L. Pearce (Baltimore, USA)
- Gerard Socie (Paris, France)
- Sophie Caillat-Zucman (Paris, France)
- Robert Soiffer (Boston, USA)
- Luca Vago (Milano, Italy)
- Bukhard Becher (Zurich, Switzerland)
- Robert Zeiser (University Medical Center Freiburg)
- Kristina Maas-Bauer, University Medical Center Freiburg
- Natalie Köhler, University Medical Center Freiburg
Selected publications
Braun LM, Giesler S, Andrieux G, Riemer R, Talvard-Balland N, Duquesne S, Ruckert T, Unger S, Kreutmair S, Zwick M, Follo M, Hartmann A, Osswald N, Melchinger W, Chapman S, Hutchinson JA, Haferkamp S, Torster L, Kott J, Gebhardt C, Hellwig D, Karantzelis N, Wallrabenstein T, Lowinus T, Yucel M, Brehm N, Rawluk J, Pfeifer D, Bronsert P, Rogg M, Mattern S, Heikenwalder M, Fusco S, Malek NP, Singer S, Schmitt-Graeff A, Ceteci F, Greten FR, Blazar BR, Boerries M, Kohler N, Duyster J, Ihorst G, Lassmann S, Keye P, Minguet S, Schadendorf D, Ugurel S, Rafei-Shamsabadi D, Thimme R, Hasselblatt P, Bengsch B, Schell C, Pearce EL, Meiss F, Becher B, Funke-Lorenz C, Placke JM, Apostolova P*, Zeiser R*. (2025) Adiponectin reduces immune checkpoint inhibitor-induced inflammation without blocking anti-tumor immunity. Cancer Cell. 43: 269-291 e19. doi: 10.1016/j.ccell.2025.01.004
Chatterjee S, Ruckert T, Martin I, Michaeli E, Buescher J, Apostolova P, Erny D, Lalioti ME, Biavasco F, Hartmann A, Runge S, Braun LM, Talvard-Balland N, Adams RC, Schmitt-Graeff A, Cook J, Wenger V, Athanassopoulos D, Hasavci D, Vallejo-Janeta AP, Blank T, Schaible P, Vinnakota JM, Zahringer A, Ganal-Vonarburg SC, Melchinger W, Pfeifer D, Kohler N, Rosshart SP, Michonneau D, Socie G, Andrieux G, Cabezas-Wallscheid N, Boerries M, Prinz M, Zeiser R. (2025) Gut microbiota-derived TMAVA is a modulator of acute CNS-GVHD. J Exp Med. 222. doi: 10.1084/jem.20242180
Maas-Bauer K, Stell AV, Yan KL, de Vega E, Vinnakota JM, Unger S, Nunez N, Norona J, Talvard-Balland N, Kossmann S, Schwan C, Miething C, Martens US, Shoumariyeh K, Nestor RP, Duquesne S, Hanke K, Rackiewicz M, Hu Z, El Khawanky N, Taromi S, Andrlova H, Faraidun H, Walter S, Pfeifer D, Follo M, Waldschmidt J, Melchinger W, Rassner M, Wehr C, Schmitt-Graeff A, Halbach S, Liao J, Hacker G, Brummer T, Dengjel J, Andrieux G, Grosse R, Tugues S, Blazar BR, Becher B, Boerries M, Zeiser R. (2024) ROCK1/2 signaling contributes to corticosteroid-refractory acute graft-versus-host disease. Nat Commun. 15: 446. doi: 10.1038/s41467-024-44703-7
Talvard-Balland N, Braun LM, Dixon KO, Zwick M, Engel H, Hartmann A, Duquesne S, Penter L, Andrieux G, Rindlisbacher L, Acerbis A, Ehmann J, Kollerer C, Ansuinelli M, Rettig A, Moschallski K, Apostolova P, Brummer T, Illert AL, Schramm MA, Cheng Y, Kottgen A, Duyster J, Menssen HD, Ritz J, Blazar BR, Boerries M, Schmitt-Graff A, Sariipek N, Van Galen P, Buescher JM, Cabezas-Wallscheid N, Pahl HL, Pearce EL, Soiffer RJ, Wu CJ, Vago L, Becher B, Kohler N, Wertheimer T, Kuchroo VK, Zeiser R. (2024) Oncogene-induced TIM-3 ligand expression dictates susceptibility to anti-TIM-3 therapy in mice. J Clin Invest. 134. doi: 10.1172/JCI177460
Talvard-Balland N, Lambert M, Chevalier MF, Minet N, Salou M, Tourret M, Bohineust A, Milo I, Parietti V, Yvorra T, Socie G, Lantz O, Caillat-Zucman S. (2024) Human MAIT cells inhibit alloreactive T cell responses and protect against acute graft-versus-host disease. JCI Insight. 9. doi: 10.1172/jci.insight.166310
Talvard-Balland N, Sutra Del Galy A, Michonneau D, Le Buanec H, Chasset F, Robin M, Peffault de Latour R, Xhaard A, Sicre de Fontbrune F, Parquet N, Duchez S, Schiavon V, Rybojad M, Bergeron-Lafaurie A, Bagot M, Bensussan A, Caillat-Zucman S, Socie G, Bouaziz JD, de Masson A. (2021) Expansion of Circulating CD49b(+)LAG3(+) Type 1 Regulatory T Cells in Human Chronic Graft-Versus-Host Disease. J Invest Dermatol. 141: 193-197 e2. doi: 10.1016/j.jid.2020.04.018
Tourret M*, Talvard-Balland N*, Lambert M, Ben Youssef G, Chevalier MF, Bohineust A, Yvorra T, Morin F, Azarnoush S, Lantz O, Dalle JH, Caillat-Zucman S. (2021) Human MAIT cells are devoid of alloreactive potential: prompting their use as universal cells for adoptive immune therapy. J Immunother Cancer. 9. doi: 10.1136/jitc-2021-003123
Ho NHJG, Talvard-Balland N, Kohler N, Zeiser R. (2025) Immune Escape of Acute Myeloid Leukemia after Transplantation. Blood Cancer Discov. 6: 168-181. doi: 10.1158/2643-3230.BCD-24-0063
Category B
Patent WO2022122961A1: Caillat-Zucman S. and Talvard-Balland N. (2021) Pending “Use of MAIT cells for controlling graft-versus-host disease”
Awards
2024 Best Poster Award at the CRC1479 Symposium - University Medical Centre Freiburg, Germany
2023 CIBSS Launchpad Grant (Cluster of Excellence CIBSS, DFG)
2021 Award for the Best Young Oral Presentation, Annual meeting of the French Society of Immunology (SFI) - Paris, France
Animal Imaging Advisory Board
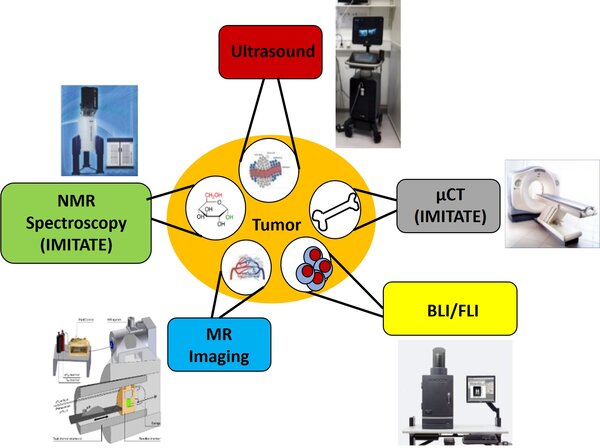
The Animal Imaging Advisory Board is a consulting service for preclinical imaging
Introduction
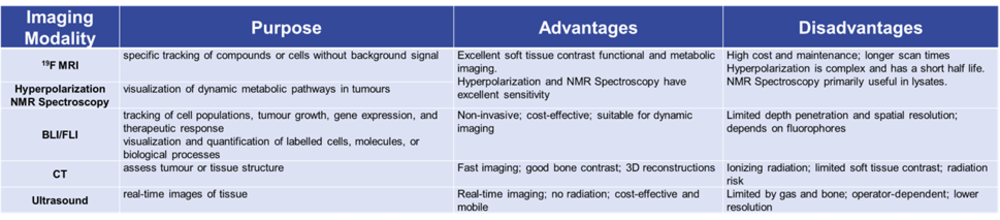
The Small Animal Imaging Advisory Board will assist the PIs of the CRC1479 initiative in selecting the most suitable imaging methods to address the scientific questions posed by their projects. Bioluminescence or fluorescence imaging (BLI/FLI), NMR spectroscopy, MR hyperpolarisation, ultrasound, and CT-based imaging will be provided. Each of these modalities has its own strengths and weaknesses and can complement one another (Table 1). Consequently, it may be challenging for individual group leaders to decide which imaging modality is best suited to their specific questions. The Advisory Board will serve as a forum to support project coordinators in defining the optimal approach for their specific requirements.
Members of the imaging advisory board/contact
The imaging advisory board consists of one project coordinator from each modality.
BLI/FLI
Dr. Nana Talvard-Balland | nana.talvard-balland@uniklinik-freiburg.de
Prof. Dr. Robert Zeiser | robert.zeiser@uniklinik-freiburg.de
MRI/US/CT
Dr. Wilfried Reichardt | wilfried.reichardt@uniklinik-freiburg.de
Procedures
The PI with interest in animal imaging needs to send the application sheet and a short abstract (about half a side) with the description of the request for an imaging application. The three members review it in a bimonthly meeting and give an advice to the applicant.