Vinnakota Laboratory
Kinase signaling and Neuroinflammation
About UsOur research group investigates the central role of TGF-β activated kinase 1 (TAK1) signaling in inflammation and cancer, aiming to understand how it shapes the balance between immune regulation, cancer progression, and safety of cancer immunotherapies. In parallel, we study the mechanisms underlying neurological toxicities of modern cancer immunotherapy, focusing on the role of microglia and myeloid cells in the central nervous system (CNS) and the role of gut–brain axis in shaping these responses. We also explore how oncogenic signaling pathways intersect with immune escape mechanisms in the context of allogeneic stem cell transplantation and relapse.
By integrating preclinical models, patient-derived systems, cellular assays and advanced imaging, our group seeks to uncover the mechanisms linking immune activation, neuroinflammation, and oncogenic signaling to identify druggable targets with translational potential.
Research Topics
TAK1 Signaling in Inflammation and Cancer
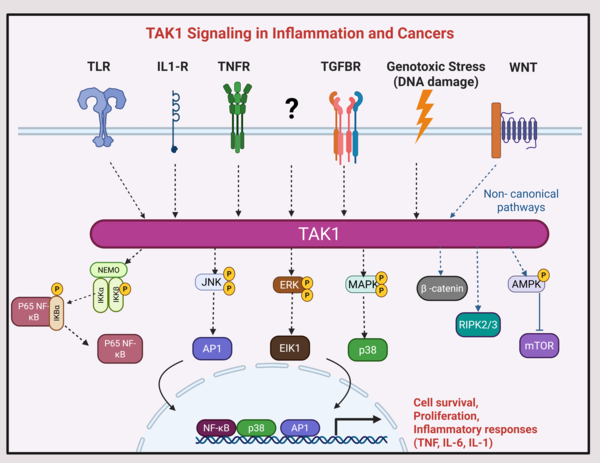
TAK1 functions as a central signaling hub linking inflammatory, stress, and developmental pathways. It activates NF-κB and MAPK pathways governing immune cell activation, proliferation, and survival, with critical roles in cancers and inflammatory diseases. Our research focuses on understanding how dysregulated TAK1 activity in immune, cancer, and tissue-resident cells drive pathological inflammation, tumor growth, and immune-related toxicities. We are particularly interested in exploring whether TAK1 inhibition can reverse these effects, offering new therapeutic avenues in cancer and inflammation.
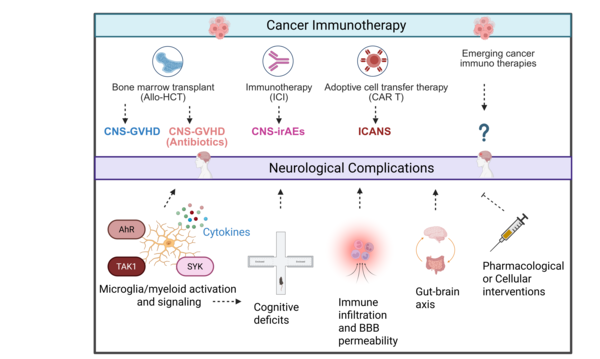
Neurological Complications of Cancer Immunotherapy
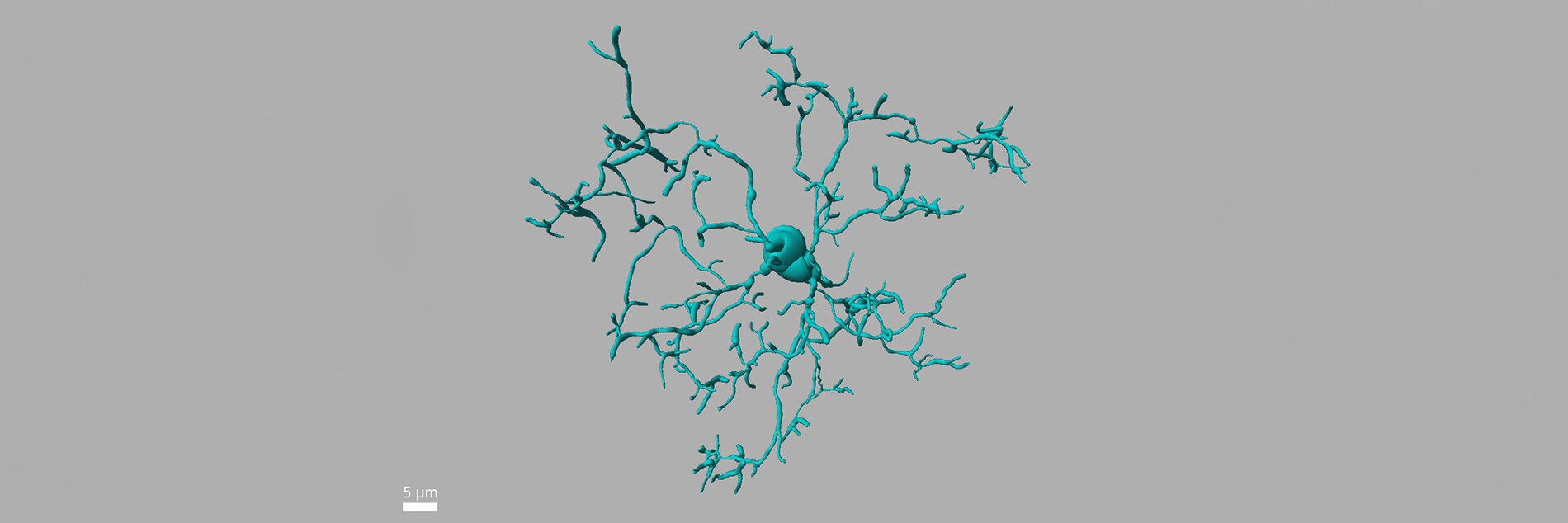
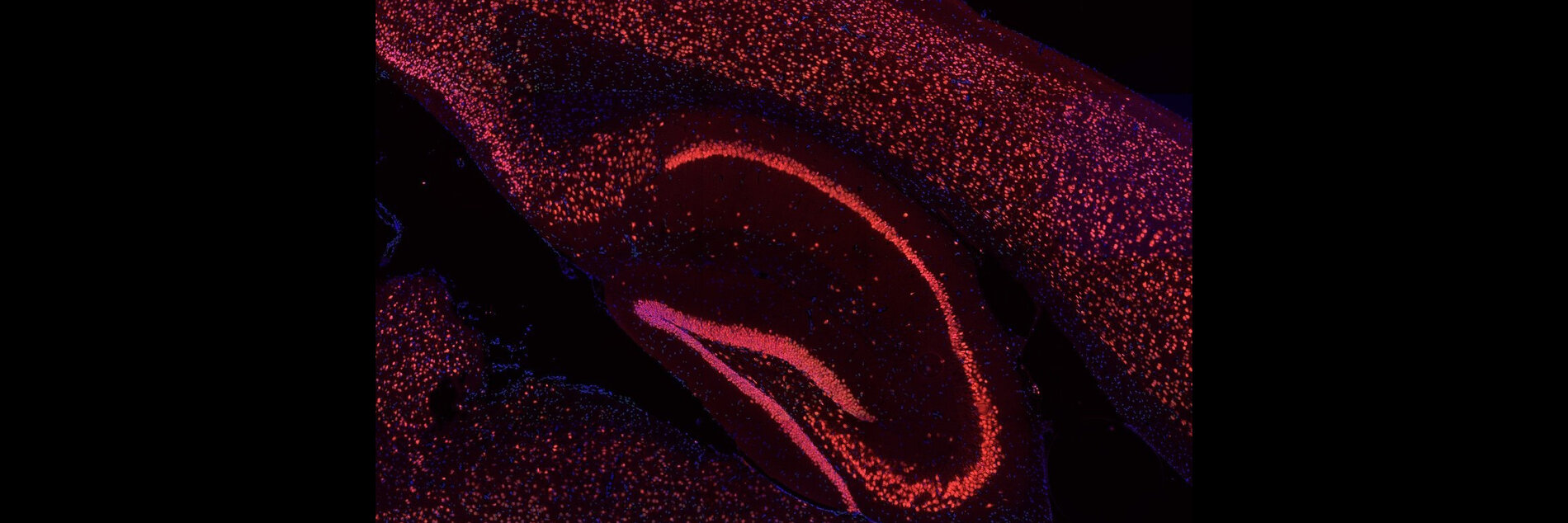
Cancer immunotherapies such as Chimeric antigen receptor (CAR) T-cells therapy, Immune checkpoint inhibition therapy (ICI), and Allogeneic stem cell transplantation (Allo-HCT) have transformed treatment outcomes but often induce severe complications in the CNS. Our research focuses on elucidating the pathomechanisms that drive these immune-related neurotoxicity’s. We investigate how aberrant microglia activation, cytokine release, endothelial dysfunction, blood–brain barrier disruption, cognitive dysfunction and gut microbiome axis contribute to CNS complications (Blood advances 2025).
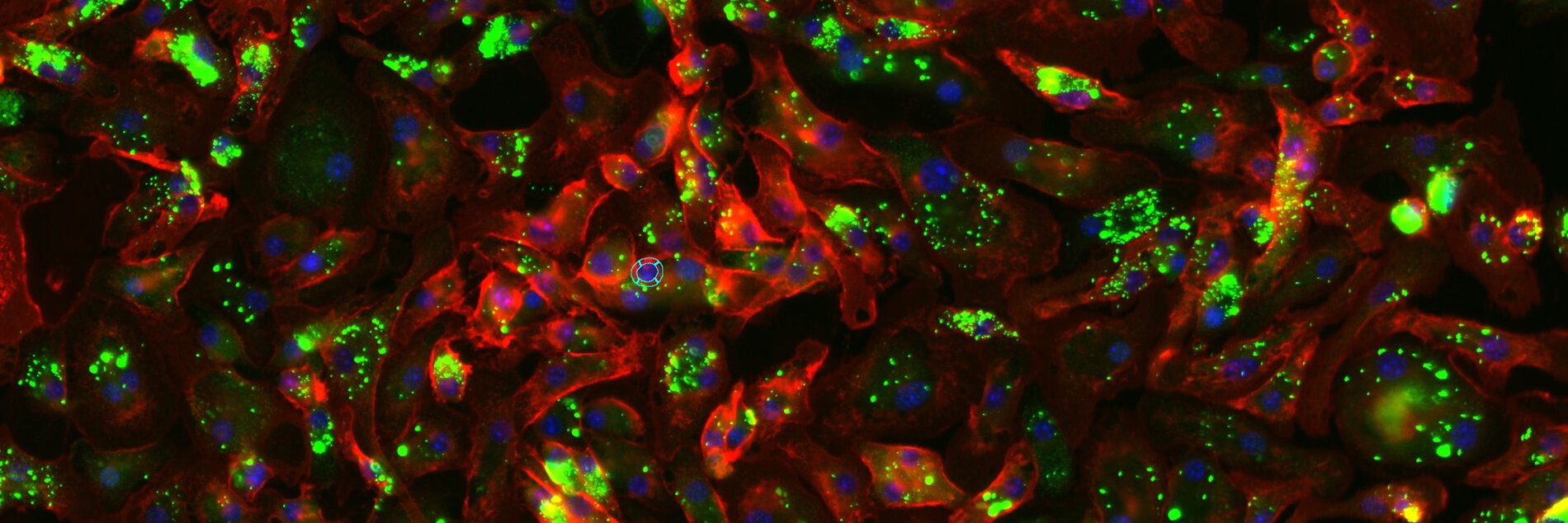
We have developed novel preclinical models that recapitulate Immune-cell-associated neurotoxicity-syndrome (ICANS), Immune-related-adverse-events (CNS-irAEs), Graft-verus-host diseases (CNS-GVHD) and demonstrated that the kinases TAK1 and Spleen tyrosine kinase (SYK) are key drivers of neuroinflammation. Targeting these kinases effectively alleviated neuroinflammatory responses and improved cognitive function in mice (Nature Cancer 2024, Science Translational Medicine 2024, JCI 2020). Building on these findings, we now apply and expand these models to emerging cancer immunotherapies, with the goal of identifying predictive biomarkers, validating therapeutic targets, advancing cellular therapy approaches to develop neuroprotective strategies. Alongside leverage kinase inhibition (TAK1, SYK, ROCK1) as a therapeutic strategy to mitigate these toxicities. Ultimately, our aim is to mitigate CNS complications while preserving or even enhancing the potent antitumor efficacy of these transformative treatments.
Oncogenic Signaling and Immune Escape Mechanisms
In parallel, we also investigate how oncogenic siCnaling promotes immune escape in Acute myeloid leukemia’s (AML) relapse after Allo-HCT. Our research examines the interactions between leukemia cells, T cells, and myeloid cells, aiming to uncover how malignant cells suppress anti-tumor immunity. The goal is to identify novel therapeutic targets that restore immune surveillance and improve the effectiveness of existing treatments
Team

Principal Investigator
Head of Vinnakota Laboratory
Dr. Janaki Manoja Vinnakota
janaki.vinnakota@uniklinik-freiburg.de
Zentrum Translationale Zellforschung (ZTZ)
Breisacher Str. 115
D- 79106 Freiburg
| Valentin Wenger | MD (ongoing) |
| Alexander Zaehringer | MD (ongoing) |
| Rachael Adams | PhD |
| Sangya Chatterjee | PhD |
| Ines Morgado | PhD |
| Madhoosudhan Suresh | PhD |
| Sara Niesen | MD |
| Dimitrios Athanassopoulos | MD |
| Enrique de Vega | B.Tech |
Publications
Giesler S, Biavasco F, Wertheimer T, Zeiser R, Vinnakota JM. Biology-driven therapies of CAR T immune effector cell-associated neurotoxicity syndrome. Immunotherapy. 2025 May;17(7):513-523. doi: 10.1080/1750743X.2025.2510893. PMID: 40438973.
Zähringer A, Morgado I, Erny D, Ingelfinger F, Gawron J, Chatterjee S, Wenger V, Schmidt D, Schwöbel L, Adams RC, Langenbach M, Hartmann A, Osswald N, Wolf J, Schlunck G, Briquez PS, Grueter K, Ruess DA, Frew I, Burk AC, Holzmüller V, Grimbacher B, Michonneau D, Andrieux G, Socié G, Kolter J, Boerries M, Follo M, Blaeschke F, Sevenich L, Prinz M, Zeiser R**, Vinnakota JM **. AhR activation mitigates graft-versus-host disease of the central nervous system by reducing microglial NF-κB signaling. Blood Adv. 2025 Jun 24;9(12):2935-2952. doi: 10.1182/bloodadvances.2024015000. PMID: 40163754.
Vinnakota JM, Adams RC, Athanassopoulos D, Schmidt D, Biavasco F, Zähringer A, Erny D, Schwabenland M, Langenbach M, Wenger V, Salié H, Cook J, Mossad O, Andrieux G, Dersch R, Rauer S, Duquesne S, Monaco G, Wolf P, Blank T, Häne P, Greter M, Becher B, Henneke P, Pfeifer D, Blazar BR, Duyster J, Boerries M, Köhler N, Chhatbar CM, Bengsch B, Prinz M, Zeiser R. Anti-PD-1 cancer immunotherapy induces central nervous system immune-related adverse events by microglia activation. Sci Transl Med. 2024 Jun 12;16(751):eadj9672. doi: 10.1126/scitranslmed.adj9672. PMID: 38865481.
Vinnakota JM, Biavasco F, Schwabenland M, Chhatbar C, Adams RC, Erny D, Duquesne S, El Khawanky N, Schmidt D, Fetsch V, Zähringer A, Salié H, Athanassopoulos D, Braun LM, Javorniczky NR, Ho JNHG, Kierdorf K, Marks R, Wäsch R, Simonetta F, Andrieux G, Pfeifer D, Monaco G, Capitini C, Fry TJ, Blank T, Blazar BR, Wagner E, Theobald M, Sommer C, Stelljes M, Reicherts C, Jeibmann A, Schittenhelm J, Monoranu CM, Rosenwald A, Kortüm M, Rasche L, Einsele H, Meyer PT, Brumberg J, Völkl S, Mackensen A, Coras R, von Bergwelt-Baildon M, Albert NL, Bartos LM, Brendel M, Holzgreve A, Mack M, Boerries M, Mackall CL, Duyster J, Henneke P, Priller J, Köhler N, Strübing F, Bengsch B, Ruella M, Subklewe M, von Baumgarten L, Gill S, Prinz M, Zeiser R. Targeting TGFβ-activated kinase-1 activation in microglia reduces CAR T immune effector cell-associated neurotoxicity syndrome. Nat Cancer. 2024 Aug;5(8):1227-1249. doi: 10.1038/s43018-024-00764-7. PMID: 38741011.
Maas-Bauer K, Stell AV*, Yan KL*, de Vega E*, Vinnakota JM*, Unger S, Núñez N, Norona J, Talvard-Balland N, Koßmann S, Schwan C, Miething C, Martens US, Shoumariyeh K, Nestor RP, Duquesne S, Hanke K, Rackiewicz M, Hu Z, El Khawanky N, Taromi S, Andrlova H, Faraidun H, Walter S, Pfeifer D, Follo M, Waldschmidt J, Melchinger W, Rassner M, Wehr C, Schmitt-Graeff A, Halbach S, Liao J, Häcker G, Brummer T, Dengjel J, Andrieux G, Grosse R, Tugues S, Blazar BR, Becher B, Boerries M, Zeiser R. ROCK1/2 signaling contributes to corticosteroid-refractory acute graft-versus-host disease. Nat Commun. 2024 Jan 10;15(1):446. doi: 10.1038/s41467-024-44703-7. PMID: 38199985.
Vinnakota JM, Zeiser R. Acute Graft-Versus-Host Disease, Infections, Vascular Events and Drug Toxicities Affecting the Central Nervous System. Front Immunol. 2021 Oct 6;12:748019. doi: 10.3389/fimmu.2021.748019. PMID: 34691059
Mathew NR*, Vinnakota JM*, Apostolova P, Erny D, Hamarsheh S, Andrieux G, Kim JS, Hanke K, Goldmann T, Chappell-Maor L, El-Khawanky N, Ihorst G, Schmidt D, Duyster J, Finke J, Blank T, Boerries M, Blazar BR, Jung S, Prinz M, Zeiser R. Graft-versus-host disease of the CNS is mediated by TNF upregulation in microglia. J Clin Invest. 2020 Mar 2;130(3):1315-1329. doi: 10.1172/JCI130272. PMID: 31846439.
Vinnakota JM, Gummadi SN. Snail represses the expression of human phospholipid scramblase 4 gene. Gene. 2016 Oct 15;591(2):433-41. doi: 10.1016/j.gene.2016.06.050. PMID: 27363667.
Vinnakota JM, Gummadi SN. Two c-Myc binding sites are crucial in upregulating the expression of human phospholipid scramblase 1 gene. Biochem Biophys Res Commun. 2016 Jan 15;469(3):412-7. doi: 10.1016/j.bbrc.2015.11.131. PMID: 26679604.
Zähringer A, Vinnakota JM, Wertheimer T, Follo M, Zeiser R. Protocol for performing and analyzing a live-cell imaging-based microglia phagocytosis assay using AI on the Olympus ScanR system. STAR Protoc. 2025 Jul 15;6(3):103846. doi: 10.1016/j.xpro.2025.103846. PMID: 40674220.
Chatterjee S, Rückert T, Martin I, Michaeli E, Buescher J, Apostolova P, Erny D, Lalioti ME, Biavasco F, Hartmann A, Runge S, Braun LM, Talvard-Balland N, Adams RC, Schmitt-Graeff A, Cook J, Wenger V, Athanassopoulos D, Hasavci D, Vallejo-Janeta AP, Blank T, Schaible P, Vinnakota JM, Zähringer A, Ganal-Vonarburg SC, Melchinger W, Pfeifer D, Köhler N, Rosshart SP, Michonneau D, Socié G, Andrieux G, Cabezas-Wallscheid N, Boerries M, Prinz M, Zeiser R. Gut microbiota-derived TMAVA is a modulator of acute CNS-GVHD. J Exp Med. 2025 Sep 1;222(9):e20242180. doi: 10.1084/jem.20242180. PMID: 40627379.
Lysandrou M, Kefala D, Vinnakota JM, Savvopoulos N, Zeiser R, Spyridonidis A. Regulatory T cell therapy for Graft-versus-Host Disease. Bone Marrow Transplant. 2025 Jul;60(7):933-939. doi: 10.1038/s41409-025-02553-x. PMID: 40240498.
Schmidt D, Endres C, Hoefflin R, Andrieux G, Zwick M, Karantzelis N, Staehle HF, Vinnakota JM, Duquesne S, Mozaffari Jovein M, Pfeifer D, Becker H, Blazar BR, Zähringer A, Duyster J, Brummer T, Boerries M, Baumeister J, Shoumariyeh K, Li J, Green AR, Heidel FH, Tirosh I, Pahl HL, Leimkühler N, Köhler N, de Toledo MAS, Koschmieder S, Zeiser R. Oncogenic Calreticulin Induces Immune Escape by Stimulating TGFβ Expression and Regulatory T-cell Expansion in the Bone Marrow Microenvironment. Cancer Res. 2024 Sep 16;84(18):2985-3003. doi: 10.1158/0008-5472.CAN-23-3553. PMID: 38885318.
Adams RC, Carter-Cusack D, Llanes GT, Hunter CR, Vinnakota JM, Ruitenberg MJ, Vukovic J, Bertolino P, Chand KK, Wixey JA, Nayler SP, Hill GR, Furlan SN, Zeiser R, MacDonald KPA. CSF1R inhibition promotes neuroinflammation and behavioral deficits during graft-versus-host disease in mice. Blood. 2024 Mar 7;143(10):912-929. doi: 10.1182/blood.2023022040. PMID: 38048572.
Isbell LK, Tschuch C, Doostkam S, Waldeck S, Andrieux G, Shoumariyeh K, Lenhard D, Schaefer HE, Reinacher PC, Bartsch I, Pantic M, Vinnakota JM, Kakkassery V, Schorb E, Scherer F, Frey AV, Boerries M, Illerhaus G, Duyster J, Schueler J, von Bubnoff N. Patient-derived xenograft mouse models to investigate tropism to the central nervous system and retina of primary and secondary central nervous system lymphoma. Neuropathol Appl Neurobiol. 2023 Apr;49(2):e12899. doi: 10.1111/nan.12899. PMID: 36879456
Ho JNHG*, Schmidt D*, Lowinus T*, Ryoo J*, Dopfer EP*, Gonzalo Núñez N, Costa-Pereira S, Toffalori C, Punta M, Fetsch V, Wertheimer T, Rittmann MC, Braun LM, Follo M, Briere C, Vinnakota JM, Langenbach M, Koppers F, Shoumariyeh K, Engel H, Rückert T, Märklin M, Holzmayer S, Illert AL, Magon F, Andrieux G, Duquesne S, Pfeifer D, Staniek J, Rizzi M, Miething C, Köhler N, Duyster J, Menssen HD, Boerries M, Buescher JM, Cabezas-Wallscheid N, Blazar BR, Apostolova P, Vago L, Pearce EL, Becher B, Zeiser R. Targeting MDM2 enhances antileukemia immunity after allogeneic transplantation via MHC-II and TRAIL-R1/2 upregulation. Blood. 2022 Sep 8;140(10):1167-1181. doi: 10.1182/blood.2022016082. PMID: 35853161
El Khawanky N, Hughes A, Yu W, Myburgh R, Matschulla T, Taromi S, Aumann K, Clarson J, Vinnakota JM, Shoumariyeh K, Miething C, Lopez AF, Brown MP, Duyster J, Hein L, Manz MG, Hughes TP, White DL, Yong ASM, Zeiser R. Demethylating therapy increases anti-CD123 CAR T cell cytotoxicity against acute myeloid leukemia. Nat Commun. 2021 Nov 8;12(1):6436. doi: 10.1038/s41467-021-26683-0. PMID: 34750374.
Hamarsheh S, Osswald L, Saller BS, Unger S, De Feo D, Vinnakota JM, Konantz M, Uhl FM, Becker H, Lübbert M, Shoumariyeh K, Schürch C, Andrieux G, Venhoff N, Schmitt-Graeff A, Duquesne S, Pfeifer D, Cooper MA, Lengerke C, Boerries M, Duyster J, Niemeyer CM, Erlacher M, Blazar BR, Becher B, Groß O, Brummer T, Zeiser R. Oncogenic KrasG12D causes myeloproliferation via NLRP3 inflammasome activation. Nat Commun. 2020 Apr 3;11(1):1659. doi: 10.1038/s41467-020-15497-1. PMID: 32246016.
** Co corresponding authors * Co-first authors
Awards
2025-2027 : Hans A Krebs Medical Scientist grant, University of Freiburg
2024-2026 : Selected for CORA Coaching for women in academia program
2025 : Jon J van Rood award, EBMT, Florence, Italy
2024 : Prize for Innovative Research Approaches in Oncology, University of Freiburg
2022 : Best oral presenter award- Med-I Retreat, University Medical Centre Freiburg,
2013-2016 : Masters Fellowship from the Ministry of Human Resource Development, IIT-Madras, Government of India.
Collaborations
International
Dean Felsher (Stanford, USA)
Munjal Acharya (UC Irvine, USA)
Melody Smith (Stanford, USA)
National
Chintan Chhatbar (Freiburg)
Daniel Erny (Freiburg)
Geoffroy Andrieux (Freiburg)
Josef Priller (Munich)
Katrin Kierdorf (Freiburg)
Lisa Sevenlich (Tubingen)
Marco Prinz (Freiburg)
Marius Schwabenland (Freiburg)
Melanie Boerries (Freiburg)
Natalie Koehler (Freiburg)
Robert Zeiser (Freiburg)
Susana Minguet (Freiburg)
Tobias Wertheimer (Freiburg)
Dr. Janaki Manoja Vinnakota
janaki.vinnakota@uniklinik-freiburg.de
ZTZ, Breisacher Strasse 115
79106 Freiburg, Germany