Zeiser Lab
Allo-ImmunregulationScientific focus
Our work can be divided into two major themes that are connected:
Cancer immune escape mechanisms and Cancer immunotherapy induced side effects
Focus 1: Cancer immune escape mechanisms
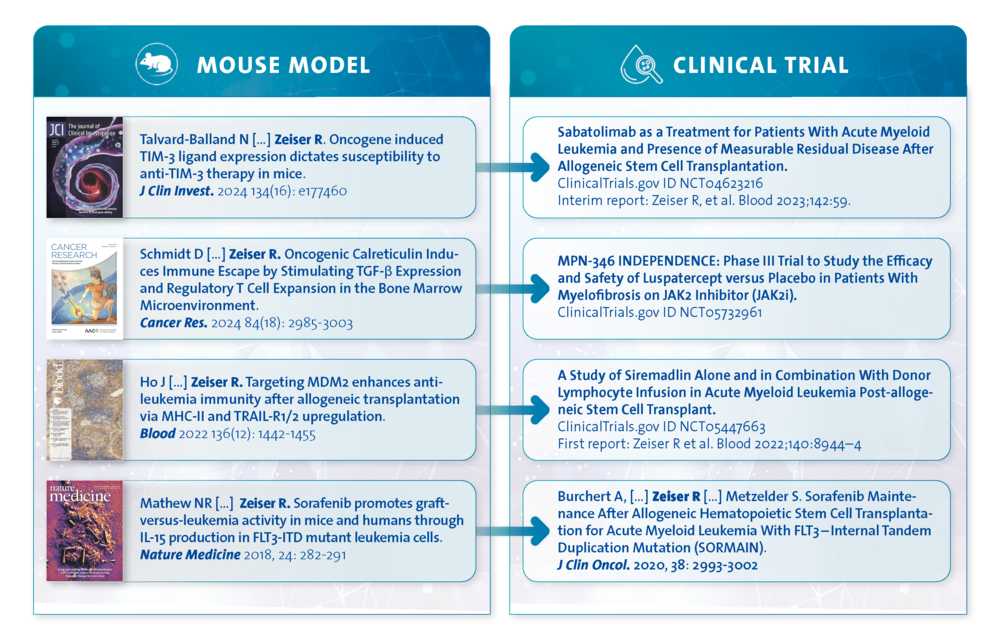
Our goal is to develop rational combinatorial treatments, which target oncogenic signalling and activate immune cells to interfere with cancer cell immune evasion. We could previously show connections between oncogenic proteins or reduced expression of tumor suppressors and immune evasion. Our preclinical studies have led to novel therapeutic concepts and corresponding clinical trials.
Preclinical studies:
FLT3-inhibition (sorafenib) induces IL-15 in FLT3-ITD AML cells (Nat Med 2018)
JAK2-V617F induces PD-L1 expression in MPN (Science Trans Med 2018)
BRAF/miR146a reduce IFNү production in melanoma (Cancer Res. 2019)
KRAS-G12D induces NLRP3/IL1ß activation in AML (Nat Comm 2020)
AML derived lactic acid reprograms T cells (Science Trans Med 2020)
Demethylating therapy enhances CAR T cell activity against AML (Nat Comm 2020)
Enhanced p53 expression causes MHC-II upregulation in leukemia (Blood 2022)
MDM2 blockade induces IL-15 production by melanoma cells (Mol Cancer Res 2023)
Oncogene induced TIM-3 ligands dictate susceptibility to anti-TIM-3 therapy (J Clin Invest 2024)
Oncogenic Calreticulin Induces Immune Escape by Stimulating TGF-β Expression and Regulatory T Cell Expansion (Cancer Res. 2024)
Corresponding clinical trials (published or ongoing):
Sorafenib reduces the relapse risk of FLT3-ITD AML after allo-HCT (JCO 2020)
Demethylating therapy enhances anti-PD1 immunotherapy for AML (Brit J Haematol 2023)
NLRP3 inflammasome activation and symptom burden in KRAS-mutated CMML patients is reverted by IL-1 blocking therapy (Cell Rep Med. 2023)
MDM2 inhibition (siremadlin) to prevent AML relapse (Clinical Trial NCT05447663, Blood 2022; 140 (Suppl.): 8944–8945)
TIM3-inhibition (sabatolimab) for AML post allo-HCT (Clinical Trial NCT04623216, Blood. 2023; 142 (Suppl 1): 59)
Focus 2: Cancer immunotherapy mediated side effects:
Immunotherapeutic interventions cause immune mediated side effects which include acute graft-versus-host disease (GVHD) after allo-HCT, Immune effector cell-associated neurotoxicity syndrome (ICANS) after CAR T cell transfer and immune related adverse events (irAEs) after immune checkpoint inhibition.
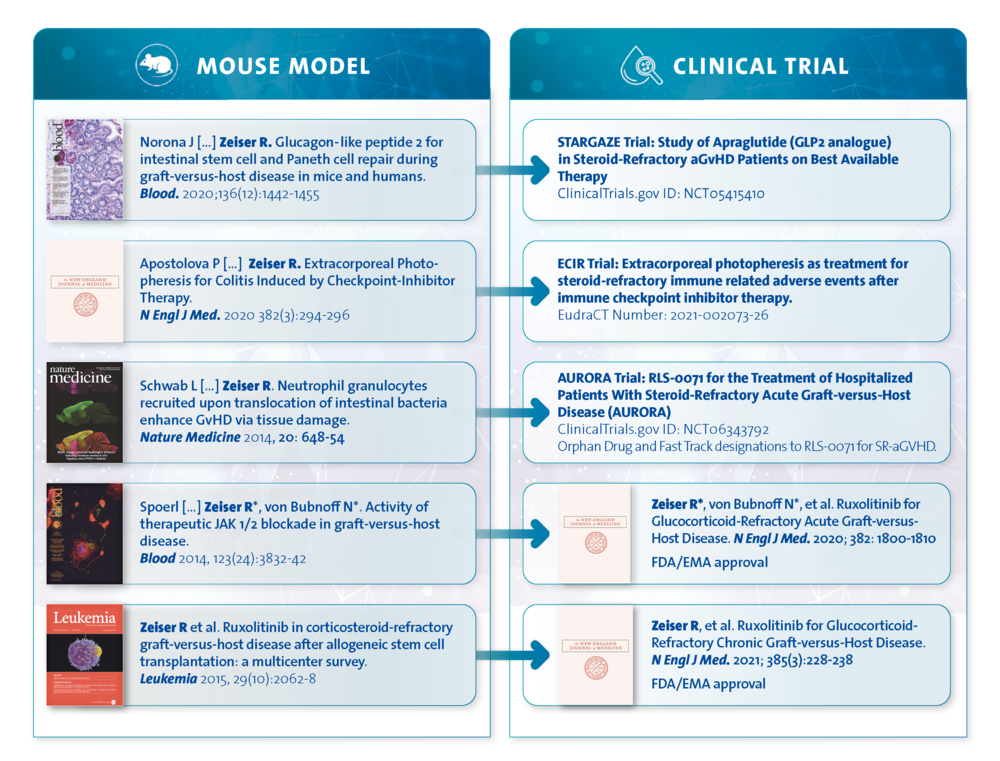
Preclinical studies:
GVHD:
GVHD is enhanced by extracellular ATP activating P2X7R (Nat Med 2010)
The Nlrp3 inflammasome regulates acute acute GVHD (JEM 2013)
Neutrophils cause tissue damage during acute GVHD (Nat Med 2014)
Ruxolitinib (JAK1/2 inhibition) reduces acute GVHD (Blood 2014)
Ruxolitinib reduces chronic GVHD (Leukemia 2015)
GLP-2 causes intestinal regeneration during aGVHD (Blood 2020)
ROCK1/2-inhibition reduces aGVHD (Nat Comm 2024)
Lipocalin-2 defines a regulatory neutrophil subset during aGVHD (Science Trans Med 2024)
ICANS:
Targeting TGFβ-activated kinase-1 activation in microglia reduces CAR T immune effector cell-associated neurotoxicity syndrome (Nature Cancer 2024)
irAEs:
Extracorporal photopheresis (ECP) for ICI-colitis (NEJM 2020)
Anti-PD-1 cancer immunotherapy induces central nervous system immune-related adverse events by microglia activation (Sci Transl Med. 2024)
Corresponding clinical trials (published or ongoing):
GVHD:
REACH2 phase-III trial; leading to EMA approval of the JAK1/2 inhibitor ruxolitinib for SR/D-acute GVHD (NEJM 2020)
REACH3 phase-III trial; leading to FDA and EMA approval of ruxolitinib for SR/D-chronic GVHD (NEJM 2021)
GLP2 (apraglutide) for intestinal acute GVHD, phase I/IIb trial (NCT05415410)
IDUNN trial, MSC vs BAT for SR acute GVHD, phase III trial (NCT04629833)
irAEs:
Extracorporal photopheresis (ECP) for ICI-colitis, phase I/IIb trial (EudraCT Number: 2021-002073-26 and Braun et al. Cancer Cell 2025)
Cancer immunotherapy translation into clinical trials
GVHD and immune related adverse events Translation into clinical trials
Team

Dr. Lukas Braun
Postdoc
Ann-Cathrin Burk
PhD Studentin
Dr. Sangya Chatterjee
Postdoc
Claudia D’Avanzo
Ärztin

Sandra Duquesne
Lab Manager
Viktor Fetsch
PhD Student
Jana Gawron
PhD Studentin
Sophie Giesler
Ärztin
Alina Hartmann
TA

Jenny Ho
Ärztin

Verena Holzmüller
PhD student

Ines Morgado de Sa
PhD Student

Dr. Wolfgang Melchinger
Postdoc
Roxane Riemer
Ärztin

Dominik Schmidt
PhD Student Molekulare Medizin

Lennard_Schwöbel
med. Doktorand
Madhusoodhanan Suresh
PhD student
Valentin Wenger
Medizinischer Doktorand
Alexander Zähringer
med. Doktorand
Vincenz-Czerny-Preis for Dr. Lukas Braun
25.10.2025 I Dr. Lukas Braun was awarded the prestigious Vincenz-Czerny-Preis by the German Society of Hematology and Medical Oncology (DGHO e.V.). The Vincenz-Czerny-Preis recognizes outstanding scientific and clinical contributions in the field of oncology. Dr. Braun received the award for his work entitled “Adiponectin reduces immune checkpoint inhibitor-induced inflammation without blocking anti-tumor immunity” (Braun LM et al., Cancer Cell, 2025). The award ceremony took place at the annual meeting of the DGHO in Cologne
“GEFI Förderpreis“, “Preis für innovative Forschungsansätze in der Onkologie“
22.10.2025 I Dr. Lukas Braun was honored with the “GEFI Förderpreis“ of the Faculty for Biology and the “Preis für innovative Forschungsansätze in der Onkologie“ of the Medical Faculty for his PhD thesis titled „Enhancing the activity of Immune Checkpoint Inhibitor therapies and controlling their side effects“. Both awards were presented during the opening ceremony of the 2025/2026 academic year at the University of Freiburg.
EBMT 2025 Annual Meeting - Jon J. van Rood Award
01.04.2025 I The Jon van Rood award for 2025 goes to Janaki Vinnakota.
The prestigious Jon J. van Rood Award honors outstanding contributions in the field of hematopoietic transplantation immunology, like graft-vs-leukaemia/tumour effects, or development of innovative therapeutic approaches based on the engineering of immune effector cells. The prize is awarded yearly by the EBMT.
The EBMT Cellular Therapy & Immunobiology Working Party (CTIWP) awarded the Jon van Rood prize to junior PI and applicant for the next CRC1479 funding period Janaki Vinnakota.The award is based on her article in Nature Cancer 2024 „Targeting TGFβ-activated kinase-1 activation in microglia reduces CAR T immune effector cell-associated neurotoxicity syndrome“

Gottfried Wilhelm Leibniz-Preis 2025
19.03.2025 I Prof. Dr. Robert Zeiser wird mit dem höchsten deutschen Forschungspreis ausgezeichnet, dem Gottfried Wilhelm Leibniz-Preis 2025 für seine herausragende Forschung in der Hämatologie und Immunologie. Das gab die Deutsche Forschungsgemeinschaft (DFG) am Mittwoch, 11. Dezember 2024 bekannt. Der Preis ist mit 2,5 Millionen Euro dotiert. Der Gottfried Wilhelm Leibniz-Preis ist der wichtigste Forschungsförderpreis in Deutschland. Ziel des Leibniz-Programms ist es, ihre Arbeitsbedingungen zu verbessern und ihre Forschungsmöglichkeiten zu erweitern.
Donnall Thomas Lecture Prize 2025
14.02.2025 I CRC 1479 scientist receives prestigious Donnall Thomas Lecture Prize.
Prof. Dr. Robert Zeiser has been honored with the esteemed Donnall Thomas Lecture Prize by the American Society for Transplantation and Cellular Therapy. This prestigious award, presented in February 2025 at the society's annual meeting in the USA, recognizes groundbreaking research in hematology. Zeiser's recognition stems from his exceptional work in translating laboratory findings into clinical trials, most notably the discovery of connections between oncogenes and immune escape mechanisms. The award holds particular significance as Prof. Zeiser is the first German recipient in 25 years, underscoring the international impact of his contributions to the field.
2024 I Prof. Dr. Robert Zeiser, awarded the DKMS Mechtild Harf Science Award 2024 for his work in allogeneic stem cell transplantation. In a ceremony held in Glasgow on 16 April, the blood cancer research community gathered to honour Professor Dr. Robert Zeiser with the DKMS Mechtild Harf Science Award 2024.
10.10.2024 I Dr. Janaki Manoja Vinnakota has received the “Preis für innovative Forschungsansätze in der Onkologie“ for her PhD dissertation on Neurological Complications Associated with Cancer Immunotherapies’’ on the 10th October 2024 at der Feierlichen Eröffnung des Akademischen Jahres, University of Freiburg.
2023 | ERC Advanced Grant für Robert Zeiser ►
2023 | Frau Dr. Jenny Ho hat am 18.10.2023 den Karl Joseph Beck Preis 2023 für Ihre Promotionsarbeit zur Rolle von p53/MDM2 in der Immunantwort gegen Leukämiezellen (AG Zeiser) erhalten.
Frau Syngia Chatterjee (AG Zeiser) hat den Young scientist Poster award (EMBO award) bei dem INTERNATIONAL SYMPOSIUM on Brain Myeloid Cells gewonnen. ►
Our work in Blood (Ho et al. Blood 140, 1167-1181, 2022) was highlighted by this Editorial:
Bleakley M, Biernacki M.
MDM2 inhibition augments GVL effect.
Blood. 2022 Sep 8;140(10):1064-1065. ►
Our work in Lancet Haematology (Zeiser R. et al. Lancet Haematol 2022 Jan;9(1):e14-e25. doi: 10.1016/S2352-3026(21)00367-7) was highlighted by this Editorial:
Paczesny S. Graft-versus-host disease treatment beyond corticosteroids in newly diagnosed patients?
Lancet Haematol. 2022 Jan;9(1):e2-e3. doi: 10.1016/S2352-3026(21)00376-8. PMID: 34971578.
https://www.sciencedirect.com/science/article/abs/pii/S2352302621003677?via%3Dihub
Prof. Dr. Robert Zeiser has received the CIBSS publication award by the Centre for Integrative Biological Signaling Studies.
- ERC Advanced
- SFB1160 (Teilprojekt)
- TRR/SFB 167 (Teilprojekt)
- SFB850 (Teilprojekt, Service Projekt)
- DFG individual grant
- Heisenberg Professorship (until 2019)
- Deutsche José Carreras Leukämie-Stiftung
- Deutsche Krebshilfe
- Wilhelm Sander-Stiftung
- SFB1479 (Speaker)
- BMBF
- Erika Pearce (MPI Freiburg / Baltimore)
- Takanori Teshima (Hokkaiko, Japan)
- Geoffrey Hill (Fred Hutchinson Cancer Research Centre, Seattle)
- Bruce Blazar (University of Minnesota)
- Pavan Bachyreddy (DFCI, Boston)
- Luca Vago (University of Milano)
- Gerald Pier (Brigham and Women's Hospital/Harvard Medical School, Boston)
- Robert Negrin (Stanford University)
- Gerard Socié (Univ. of Paris)
- Mathew Cooper (Univ. of Queensland, Brisbane)
- Dean Felsher (Stanford University)
- Georg Häcker (Microbiology, Freiburg)
- Marco Prinz (Neuropathology, Freiburg)
- Andreas Beilhack (Hematology/Oncology, Würzburg)
- Tilman Brummer (IMMZ, Freiburg)
- Tim Lämmermann (MPI Freiburg)
- Melanie Börries (Medical informatics, Freiburg)
- Linda Thompson (University of Oklahoma Health Sciences Center, Oklahoma)
- Olaf Groß (Neuropathology, Freiburg)
- Miriam Erlacher (Pediatrics, Freiburg)
- Bertram Bengsch (Med II, Freiburg)
- Ernst Holler (Hematology/Oncology, Regensburg)
- Susana Minguet (Biology/BIOSS, Freiburg)
- Toni Cathomen (Transfusion and Gene Therapy, Freiburg)
- Francis Ayuk (Univ. Hospital KMT, Hamburg)
- Ian Frew (Hematology/Oncology, Freiburg)
- Lena Illert (Hematology/Oncology, Freiburg)
- Justus Duyster (Hematology/Oncology, Freiburg)
- Oliver Schilling (Pathology, Freiburg)
- Mathias Heikenwälder (DKFZ Heidelberg)
Gawron J, Czech M, Rückert T, Holzmüller V, Andreev G, Burk AC, Hartmann A, Chatterjee S, Andrieux G, Marquard FE, Baur AS, Stell AV, Krausz M, Braun LM, Osswald N, Melchinger W, Wertheimer T, Proano-Vasco AI, Maas-Bauer K, Schmitt-Graeff A, Boerries M, Köhler N, Ayuk FA, Schell C, Quante M, Zeiser R. Gastrin for the treatment of acute graft-versus-host disease of the stomach. Blood. 2026 Jan 9:blood.2025031080. doi: 10.1182/blood.2025031080.
Fetsch V, Schwöbel LF, Ozyerli-Goknar E PhD, Stell AV, Punta M, Plenge T, Klaus T, Gupta MK, Andrieux G, Shoumariyeh K, Pfeiffer S, Corrales E, Schlenke L, Baniadam H, Brandl SM, Andreis M, Remen M, Hartmann A, Grueter K, Zwick M, Köhler N, Kuban M, Metzger E, Rummelt C, Duyster J, Börries M, Hofmann M, Färber J, Braun LM, Zähringer A, Lübbert M, Toffalori C, Vago L, Heidel FH, Minguet S, Apostolova P, Feuchtinger T, Maas-Bauer K, Blaeschke F, Kühn MWM, Timmers M, Wertheimer T, Perner F, Zeiser R. Menin inhibition enhances graft-versus-leukemia effects by T-cell activation and endogenous retrovirus induction in AML. Blood. 2026; 147(5):584-601 doi: 10.1182/blood.2025029712.
Highlighted by: Chakraverty R. Menin inhibitors: a 2-in-1 defense versus AML immune evasion. Blood (2026) 147 (5): 482–483.
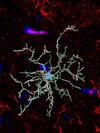
Zähringer A, Vinnakota JM, Wertheimer T, Saalfrank P, Follo M, Ingelfinger F, Zeiser R. AIstain: Enhancing microglial phagocytosis analysis through deep learning. Cell Rep Methods. 2025 Oct 17:101207. doi: 10.1016/j.crmeth.2025.101207.
Highlighted on the Cover of the journal.
Chatterjee S, Rückert T, Martin I, Michaeli E, Buescher J, Apostolova P, Erny D, Lalioti ME, Biavasco F, Hartmann A, Runge S, Braun LM, Talvard-Balland N, Adams RC, Schmitt-Graeff A, Cook J, Wenger V, Athanassopoulos D, Hasavci D, Vallejo-Janeta AP, Blank T, Schaible P, Vinnakota JM, Zähringer A, Ganal-Vonarburg SC, Melchinger W, Pfeifer D, Köhler N, Rosshart SP, Michonneau D, Socié G, Andrieux G, Cabezas-Wallscheid N, Boerries M, Prinz M, Zeiser R.
Gut microbiota-derived TMAVA is a modulator of acute CNS-GVHD.
J Exp Med. 2025; 222(9):e20242180. doi: 10.1084/jem.20242180.
Highlighted by: Kenison JE, Quintana FJ. Antibiotics unleash neuroinflammation. J Exp Med. 2025 Sep 1;222(9):e20251233

Zeiser R, Russo D, Ram R, Hashmi SK, Chakraverty R, Middeke JM, Musso M, Giebel S, Uzay A, Langmuir P, Hamad N, Burock K, Gowda M, Stefanelli T, Lee SJ, Teshima T, Locatelli F. Ruxolitinib in Patients With Corticosteroid-Refractory or Corticosteroid-Dependent Chronic Graft-Versus-Host Disease: 3-Year Final Analysis of the Phase III REACH3 Study.
J Clin Oncol. 2025 Jun 25:JCO2402477. doi: 10.1200/JCO-24-02477.

Brehm N, Biavasco F, Clausen J, Jung J, Maas-Bauer K, Wäsch R, Verbeek M, Nuernbergk C, Ihorst G, Seropian S, Finke J, Gowda L, Sidlik Muskatel R, Peffault de Latour R, Socie G, Wehr C, Michonneau D, Zeiser R. Teduglutide for treatment-refractory severe intestinal acute graft-versus-host disease - a multicenter survey.
Bone Marrow Transplant. 2025 Jun;60(6):873-878. doi: 10.1038/s41409-025-02586-2.

Braun LM, Giesler S, Andrieux G, Riemer R, Talvard-Balland N, Duquesne S, Rückert T, Unger S, Kreutmair S, Zwick M, Follo M, Hartmann A, Osswald N, Melchinger W, Chapman S, Hutchinson JA, Haferkamp S, Torster L, Kött J, Gebhardt C, Hellwig D, Karantzelis N, Wallrabenstein T, Lowinus T, Yücel M, Brehm N, Rawluk J, Pfeifer D, Bronsert P, Rogg M, Mattern S, Heikenwälder M, Fusco S, Malek NP, Singer S, Schmitt-Graeff A, Ceteci F, Greten FR, Blazar BR, Boerries M, Köhler N, Duyster J, Ihorst G, Lassmann S, Keye P, Minguet S, Schadendorf D, Ugurel S, Rafei-Shamsabadi D, Thimme R, Hasselblatt P, Bengsch B, Schell C, Pearce EL, Meiss F, Becher B, Funke-Lorenz C, Placke JM, Apostolova P, Zeiser R. Adiponectin reduces immune checkpoint inhibitor-induced inflammation without blocking anti-tumor immunity.
Cancer Cell. 2025 Feb 10;43(2):269-291.e19. doi: 10.1016/j.ccell.2025.01.004.
Schmidt D, Endres C, Hoefflin R, Andrieux G, Zwick M, Karantzelis N, Staehle HF, Vinnakota JM, Duquesne S, Mozaffari Jovein M, Pfeifer D, Becker H, Blazar BR, Zähringer A, Duyster J, Brummer T, Boerries M, Baumeister J, Shoumariyeh K, Li J, Green AR, Heidel FH, Tirosh I, Pahl HL, Leimkühler N, Köhler N, de Toledo MAS, Koschmieder S, Zeiser R. Oncogenic Calreticulin Induces Immune Escape by Stimulating TGF-β Expression and Regulatory T Cell Expansion in the Bone Marrow Microenvironment.
Cancer Res. 2024 Jun 17. doi: 10.1158/0008-5472

Talvard-Balland N, Braun LM, Dixon KO, Zwick M, Engel H, Hartmann A, Duquesne S, Penter L, Andrieux G, Rindlisbacher LS, Acerbis A, Ehmann J, Köllerer C, Ansuinelli M, Rettig A, Moschallski K, Apostolova P, Brummer T, Illert AL, Schramm MA, Cheng Y, Köttgen A, Duyster J, Menssen HD, Ritz J, Blazar BR, Boerries M, Schmitt Graeff A, Sariipek N, van Galen P, Buescher JM, Cabezas-Wallscheid N, Pahl HL, Pearce EL, Soiffer RJ, Wu CJ, Vago L, Becher B, Köhler N, Wertheimer T, Kuchroo VK, Zeiser R. Oncogene induced TIM-3 ligand expression dictates susceptibility to anti-TIM-3 therapy in mice.
J Clin Invest. 2024 Jun 25:e177460. doi: 10.1172/JCI177460.
Vinnakota JM, Adams RC, Athanassopoulos D, Schmidt D, Biavasco F, Zähringer A, Erny D, Schwabenland M, Langenbach M, Wenger V, Salié H, Cook J, Mossad O, Andrieux G, Dersch R, Rauer S, Duquesne S, Monaco G, Wolf P, Blank T, Häne P, Greter M, Becher B, Henneke P, Pfeifer D, Blazar BR, Duyster J, Boerries M, Köhler N, Chhatbar CM, Bengsch B, Prinz M, Zeiser R. Anti-PD-1 cancer immunotherapy induces central nervous system immune-related adverse events by microglia activation.
Science Translational Medicine 2024 Jun 12;16(751):eadj9672. doi: 10.1126/scitranslmed.adj9672. Epub 2024 Jun 12. PMID: 38865481.
Vinnakota JM, Biavasco F, Schwabenland M, Chhatbar C, Adams RC, Erny D, Duquesne S, El Khawanky N, Schmidt D, Fetsch V, Zähringer A, Salié H, Athanassopoulos D, Braun LM, Javorniczky NR, Ho JNHG, Kierdorf K, Marks R, Wäsch R, Simonetta F, Andrieux G, Pfeifer D, Monaco G, Capitini C, Fry TJ, Blank T, Blazar BR, Wagner E, Theobald M, Sommer C, Stelljes M, Reicherts C, Jeibmann A, Schittenhelm J, Monoranu CM, Rosenwald A, Kortüm M, Rasche L, Einsele H, Meyer PT, Brumberg J, Völkl S, Mackensen A, Coras R, von Bergwelt-Baildon M, Albert NL, Bartos LM, Brendel M, Holzgreve A, Mack M, Boerries M, Mackall CL, Duyster J, Henneke P, Priller J, Köhler N, Strübing F, Bengsch B, Ruella M, Subklewe M, von Baumgarten L, Gill S, Prinz M, Zeiser R. Targeting TGFβ-activated kinase-1 activation in microglia reduces CAR T immune effector cell-associated neurotoxicity syndrome.
Nature Cancer. 2024 May 13. doi: 10.1038/s43018-024-00764-7.
Highlighted by: Targeting TAK1 in microglia to treat CAR T cell neurotoxicity. Nature Cancer. 2024, 5(8): 1143-1144.
Czech M*, Schneider S*, Peltokangas N*, El Khawanky N, Ghimire S, Andrieux G, Hülsdünker J, Krausz M, Proietti M, Braun LM, Rückert T, Langenbach M, Schmidt D, Martin I, Wenger V, de Vega E, Haring E, Pourjam M, Pfeifer D, Schmitt-Graeff A, Grimbacher B, Aumann K, Kircher B, Tilg H, Raffatellu M, Thiele Orberg E, Häcker G, Duyster J, Köhler N, Holler E, Nachbaur D, Boerries M, Gerner RR*, Grün D*, Zeiser R*. Lipocalin-2 expression identifies an intestinal regulatory neutrophil population during acute graft-versus-host disease.
Science Transl Med. 2024 Feb 21;16(735):eadi1501. doi: 10.1126/scitranslmed.adi1501.
Highlighted by:
Markey K. Lipocalin-2: a novel therapy for GVHD. Trends in Immunology 2024
Maas-Bauer K, Stell AV, Yan KL, de Vega E, Vinnakota JM, Unger S, Núñez N, Norona J, Talvard-Balland N, Koßmann S, Schwan C, Miething C, Martens US, Shoumariyeh K, Nestor RP, Duquesne S, Hanke K, Rackiewicz M, Hu Z, El Khawanky N, Taromi S, Andrlova H, Faraidun H, Walter S, Pfeifer D, Follo M, Waldschmidt J, Melchinger W, Rassner M, Wehr C, Schmitt-Graeff A, Halbach S, Liao J, Häcker G, Brummer T, Dengjel J, Andrieux G, Grosse R, Tugues S, Blazar BR, Becher B, Boerries M, Zeiser R. ROCK1/2 signaling contributes to corticosteroid-refractory acute graft-versus-host disease. Nature Commun. 2024 Jan 10;15(1):446.
Apostolova P, Kreutmair S, Toffalori C, Punta M, Unger S, Burk AC, Wehr C, Maas-Bauer K, Melchinger W, Haring E, Hoefflin R, Shoumariyeh K, Hupfer V, Lauer EM, Duquesne S, Lowinus T, Gonzalo Núñez N, Alberti C, da Costa Pereira S, Merten CH, Power L, Weiss M, Böke C, Pfeifer D, Marks R, Bertz H, Wäsch R, Ihorst G, Gentner B, Duyster J, Boerries M, Andrieux G, Finke J, Becher B, Vago L, Zeiser R.
Phase II trial of hypomethylating agent combined with nivolumab for acute myeloid leukaemia relapse after allogeneic haematopoietic cell transplantation-Immune signature correlates with response.
Br J Haematol. 2023 Aug 4. doi: 10.1111/bjh.19007.
Langenbach M, Giesler S, Richtsfeld S, da Costa-Pereira S, Rindlisbacher L, Wertheimer T, Braun LM, Andrieux G, Duquesne S, Pfeifer D, Woessner NM, Menssen HD, Taromi S, Duyster J, Boerries M, Brummer T, Blazar BR, Minguet S, Turko P, Levesque MP, Becher B, Zeiser R. MDM2 inhibition enhances immune checkpoint inhibitor efficacy by increasing IL-15 and MHC class II production. Mol Cancer Res. 2023 Apr 18:MCR-22-0898. doi: 10.1158/1541-7786.MCR-22-0898.
Ho JNHG, Schmidt D, Lowinus T, Ryoo J, Dopfer EP, Gonzalo Núñez N, Costa-Pereira S, Toffalori C, Punta M, Fetsch V, Wertheimer T, Rittmann MC, Braun LM, Follo M, Briere C, Vinnakota JM, Langenbach M, Koppers F, Shoumariyeh K, Engel H, Rückert T, Märklin M, Holzmayer S, Illert AL, Magon F, Andrieux G, Duquesne S, Pfeifer D, Staniek J, Rizzi M, Miething C, Köhler N, Duyster J, Menssen HD, Boerries M, Buescher JM, Cabezas-Wallscheid N, Blazar BR, Apostolova P, Vago L, Pearce EL, Becher B, Zeiser R.
Targeting MDM2 enhances anti-leukemia immunity after allogeneic transplantation via MHC-II and TRAIL-R1/2 upregulation.
Blood 140, 1167-1181 (2022). doi: 10.1182/blood.2022016082
Zeiser R, Socié G, Schroeder MA, Abhyankar S, Vaz CP, Kwon M, Clausen J, Volodin L, Giebel S, Chacon MJ, Meyers G, Ghosh M, Deeren D, Sanz J, Morariu-Zamfir R, Arbushites M, Lakshminarayanan M, Barbour AM, Chen YB. Efficacy and safety of itacitinib versus placebo in combination with corticosteroids for initial treatment of acute graft-versus-host disease (GRAVITAS-301): a randomised, multicentre, double-blind, phase 3 trial.
Lancet Haematol. 2022 Jan;9(1):e14-e25. doi: 10.1016/S2352-3026(21)00367-7.
El Khawanky N, Hughes A, Yu W, Myburgh R, Matschulla T, Taromi S, Aumann K, Clarson J, Vinnakota JM, Shoumariyeh K, Miething C, Lopez AF, Brown MP, Duyster J, Hein L, Manz MG, Hughes TP, White DL, Yong ASM, Zeiser R. Demethylating therapy increases anti-CD123 CAR T cell cytotoxicity against acute myeloid leukemia.
Nat Commun. 2021 Nov 8;12(1):6436.
Taromi S, Firat E, Simonis A, Braun LM, Apostolova P, Elze M, Passlick B, Schumacher A, Lagies S, Frey A, Schmitt-Graeff A, Burger M, Schmittlutz K, Follo M, von Elverfeldt D, Zhu X, Kammerer B, Diederichs S, Duyster J, Manz MG, Niedermann G, Zeiser R. Enhanced AC133-specific CAR T cell therapy induces durable remissions in mice with metastatic small cell lung cancer.
Cancer Lett. 2022 Jul 10;538:215697
Zeiser R, Polverelli N, Ram R, Hashmi SK, Chakraverty R, Middeke JM, Musso M, Giebel S, Uzay A, Langmuir P, Hollaender N, Gowda M, Stefanelli T, Lee SJ, Teshima T, Locatelli F; REACH3 Investigators. Ruxolitinib for Glucocorticoid-Refractory Chronic Graft-versus-Host Disease.
N Engl J Med. 2021 Jul 15;385(3):228-238. doi: 10.1056/NEJMoa2033122. PMID: 34260836.
Osswald L, Hamarsheh S, Uhl FM, Andrieux G, Klein C, Dierks C, Duquesne S, Braun LM, Schmitt-Graeff A, Duyster J, Boerries M, Brummer T, Zeiser R.
Oncogenic KrasG12D Activation in the Nonhematopoietic Bone Marrow Microenvironment Causes Myelodysplastic Syndrome in Mice.
Mol Cancer Res. 2021 Jun 4. doi: 10.1158/1541-7786.MCR-20-0275.
Uhl FM, Chen S, O'Sullivan D, Edwards-Hicks J, Richter G, Haring E, Andrieux G, Halbach S, Apostolova P, Büscher J, Duquesne S, Melchinger W, Sauer B, Shoumariyeh K, Schmitt-Graeff A, Kreutz M, Lübbert M, Duyster J, Brummer T, Boerries M, Madl T, Blazar BR, Groß O, Pearce EL, Zeiser R.
Metabolic reprogramming of donor T cells enhances graft-versus-leukemia effects in mice and humans.
Science Translational Medicine 2020 Oct 28;12(567):eabb8969.
Norona J, Apostolova P, Schmidt D, Ihlemann R, Reischmann N, Taylor G, Köhler N, de Heer J, Heeg S, Andrieux G, Siranosian BA, Schmitt-Graeff A, Pfeifer D, Catalano A, Frew I, Proietti M, Grimbacher B, Bulashevska A, Bhatt AS, Brummer T, Clauditz TS, Zabelina T, Kroeger N, Blazar BR, Boerries M, Ayuk F, Zeiser R.
Glucagon like peptide-2 for Intestinal stem cell and Paneth cell repair during graft-versus-host disease in mice and humans.
Blood. 2020; 136: 1442-1455.
Highlighted in: Chao N. Blood 2020
Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, Szer J, Wagner EM, Zuckerman T, Mahuzier B, Xu J, Wilke C, Gandhi KK, Socié G, for the REACH2 Trial Group.
Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease.
The New England Journal of Medicine 2020 May 7;382(19):1800-1810
Highlighted in Chao N. Finally, a Successful Randomized Trial for GVHD. N Engl J Med. 2020
R Mathew N, Vinnakota JM, Apostolova P, Erny D, Hamarsheh S, Andrieux G, Kim JS, Hanke K, Goldmann T, Chappell-Maor L, El-Khawanky N, Ihorst G, Schmidt D, Duyster J, Finke J, Blank T, Boerries M, Blazar BR, Jung S, Prinz M, Zeiser R.
Graft-versus-host disease of the CNS is mediated by TNF upregulation in microglia.
J Clin Invest. 2020 Mar 2;130(3):1315-1329
Hülsdünker J, Thomas OS, Haring E, Unger S, Gonzalo Núñez N, Tugues S, Gao Z, Duquesne S, Cywes-Bentley C, Oyardi O, Kirschnek S, Schmitt-Graeff A, Pabst O, Koenecke C, Duyster J, Apostolova P, Blaser MJ, Becher B, Pier GB, Häcker G, Zeiser R.
Immunization against poly-N-acetylglucosamine reduces neutrophil activation and GVHD while sparing microbial diversity.
Proc Natl Acad Sci U S A. 2019 Oct 8;116(41):20700-20706
Prestipino A, Emhardt A, Aumann K, O´Sullivan D, Gorantla SP, Duquesne S, Melchinger W, Braun L, Vuckovic S, Boerries M, Busch H, Halbach S, Pennisi S, Poggio T, Apostolova P, Veratti P, Hettich M, Niedermann G, Bartholomä M, Shoumariyeh K, Jutzi J, Wehrle J, Dierks C, Becker H, Schmitt-Graeff A, Follo M, Pfeifer D, Rohr J, Fuchs S, Ehl S, Hartl FA, Minguet S, Miething C, Heidel F, Kröger N, Triviai I, Brummer T, Finke J, Illert AL, Ruggiero E, Bonini C, Duyster J, Pahl HL, Lane SW, Hill GR, Blazar BR, Bubnoff N, Pearce EL, Zeiser R.
Oncogenic JAK2V617F causes PD-L1 expression mediating immune-escape in myeloproliferative neoplasms
Science Translational Medicine 10, eaam7729 2018
Mathew NR, Baumgartner F, Braun L, David O´Sullivan, Thomas S, Waterhouse M, Müller TA, Hanke K, Taromi S, Apostolova P, Illert AL, Melchinger W, Duquesne S, Schmitt-Graeff A, Osswald L, Yan K-L., Weber A, Tugues S, Spath S, Pfeifer D, Follo M, Claus R, Lübbert M, Rummelt C, Bertz H, Wäsch R, Haag J, Schmidts A, Schultheiss M, Bettinger M, Thimme R, Ullrich E, Tanriver Y, Vuong GL, Arnold R, Hemmati P, Wolf D, Ditschkowski M, Jilg C, Wilhelm K, Leiber C, Gerull S, Halter J, Lengerke C, Pabst T, Schroeder T, Kobbe G, Rösler W, Doostkam S, Meckel S, Stabla K, Metzelder SK, Halbach S, Brummer T, Hu Z, Dengjel J, Hackanson B, Schmid C, Holtick U, Scheid C, Spyridonidis A, Stölzel F, Ordemann F, Müller LP, Sicre-de-Fontbrune F, Ihorst G, Kuball J, Ehlert JE, Feger D, Wagner EV, Cahn JY, Schnell J, Kuchenbauer F, Bunjes D, Chakraverty R, Richardson S, Gill S, Kröger N, Ayuk F, Vago L, Ciceri F, Müller AM, Kondo T, Teshima T, Klaeger S, Kuster B, Kim D, Weisdorf D, van der Velden W, Dörfel D, Bethge W, Hilgendorf I, Hochhaus A, Andrieux G, Börries M, Busch H, Magenau J, Reddy P, Labopin M, H. Antin J, Henden AS, Hill GR, Kennedy GA, Bar M, Sarma A, McLornan D, Mufti G, Oran B, Rezvani K, Sha O, Negrin RS, Nagler A, Prinz M, Burchert A, Neubauer A, Beelen D, Mackensen A, von Bubnoff N, Herr W, Becher B, Socié G, Caligiuri MA, Ruggiero E, Bonini C, Häcker G, Duyster J, Finke J, Pearce E, Blazar BR, Zeiser R.
Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD mutant leukemia cells.
Nature Medicine 2018 Mar;24(3):282-291
Hülsdünker J, Ottmüller KJ, Neeff HP, Koyama M, Gao Z, Thomas OS, Follo M, Al-Ahmad A, Prinz G, Duquesne S, Dierbach H, Kirschnek S, Lämmermann T, Blaser MJ, Fife BT, Blazar BR, Beilhack A, Hill GR, Häcker G, Zeiser R.
Neutrophils provide cellular communication between ileum and mesenteric lymph nodes at graft-versus-host disease onset.
Blood. 2018 Apr 19;131(16):1858-1869.
Highlighted in Martin PJ. Blood. 2018;131(16):1774-1775
Stickel N, Hanke K, Marschner D, Prinz G, Köhler M, Melchinger W, Pfeifer D, Schmitt-Graeff A, Brummer T, Heine A, Brossart P, Wolf D, von Bubnoff N, Finke J, Duyster J, Ferrara J, Salzer U, Zeiser R. MicroRNA-146a reduces MHC-II expression via targeting JAK/STAT signaling in dendritic cells after stem cell transplantation.
Leukemia. 2017 Dec;31(12):2732-2741
Andrlová H, Mastroianni J, Madl J, Kern JS, Melchinger W, Dierbach H, Wernet F, Follo M, Technau-Hafsi K, Has C, Mittapalli VR, Idzko M, Herr R, Brummer T, Ungefroren H, Busch H,
Boerries M, Narr A, Ihorst G, Vennin C, Schmitt-Graeff A, Minguet S, Timpson P, Duyster J, Meiss F, Römer W, Zeiser R. Biglycan expression in the melanoma microenvironment promotes invasiveness via increased tissue stiffness inducing integrin-β1 expression.
Oncotarget. Apr 17. doi: 10.18632/oncotarget.17160, 2017
Schönle A, Hartl FA, Mentzel J, Nöltner T, Rauch KS, Prestipino A, Wohlfeil SA, Apostolova P, Hechinger AK, Melchinger W, Fehrenbach K, Guadamillas MC, Follo M, Prinz G, Ruess AK, Pfeifer D, Del Pozo MA, Schmitt-Graeff A, Duyster J, Hippen KI, Blazar BR, Schachtrup K, Minguet S, Zeiser R. Caveolin-1 regulates TCR signal strength and regulatory T cell differentiation into alloreactive T cells.
Blood 127:1930-9, 2016
Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, Spoerl S, Ditschkowski M, Ecsedi M, Sockel K, Ayuk F, Ajib S, de Fontbrune FS, Na IK, Penter L, Holtick U, Wolf D, Schuler E, Meyer E, Apostolova P, Bertz H, Marks R, Lübbert M, Wäsch R, Scheid C, Stölzel F, Ordemann R, Bug G, Kobbe G, Negrin R, Brune M, Spyridonidis A, Schmitt-Gräff A, van der Velden W, Huls G, Mielke S, Grigoleit GU, Kuball J, Flynn R, Ihorst G, Du J, Blazar BR, Arnold R, Kröger N, Passweg J, Halter J, Socié G, Beelen D, Peschel C, Neubauer A, Finke J, Duyster J, von Bubnoff N. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multi-center survey.
Leukemia 29: 2062-8, 2015
Hechinger AK, Smith BA, Flynn R, Hanke K, McDonald-Hyman C, Taylor PA, Pfeifer D, Hackanson B, Leonhardt F, Prinz G, Dierbach H, Schmitt-Graeff A, Kovarik J, Blazar BR, Zeiser R. Therapeutic activity of multiple common gamma chain cytokine inhibition in acute and chronic GvHD.
Blood 125: 570-80, 2015
Highlighted in Pérez-Simón JA. Blood 125: 424-6. 2015
Schwab, L, Goroncy, L, Palaniyandi, S., Gautam, S., Triantafyllopoulou, A., Mocsai, A, Reichardt, W., Karlsson, FJ, Radhakrishnan, SV, Hanke, K, Schmitt-Graeff, A, Freudenberg, M, von Loewenich, FD, Wolf, P, Leonhardt, F, Baxan, N, Pfeifer, D, Schmah, O, Schönle, A, Martin, SF, Mertelsmann, R, Duyster, J, Finke, J., Prinz, M., Henneke, P., Häcker, H., Hildebrandt, G.C, Häcker, G, Zeiser, R. Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance GvHD via tissue damage.
Nature Medicine 20: 648-54, 2014
Highlighted in Kugelberg E. Nature Reviews Immunology, 2014
Yaktapour N, Meiss F, Mastroianni J, Zenz T, Andrlova H, Mathew NR, Claus R, Hutter B, Fröhling S, Brors B, Pfeifer D, Pantic M, Bartsch I, Spehl TS, Meyer PT, Duyster J, Zirlik K, Brummer T, Zeiser R. BRAF inhibitor-associated ERK activation drives development of chronic lymphocytic leukemia.
J Clin Invest. 124: 5074-84, 2014
Highlighted in Wu C.J. J Clin Invest. 124:4681-3, 2014
Jankovic D, Ganesan J, Bscheider M, Stickel N, Weber F, Guarda G, Follo M, Pfeifer D, Tardivel A, Ludigs K, Bouazzaoui A, Kerl K, Fischer J, Haas T, Schmitt-Gräff A, Manoharan A, Müller L, Finke J, Martin S, Gorka O, Peschel C, Ruland J, Idzko M, Duyster J, Holler E, French LE, Poeck H*, Contassot E, Zeiser R. The Nlrp3-inflammasome regulates acute graft-versus-host disease.
J Exp Med 210:1899-910, 2013
Leonhardt F, Grundmann S, Behe M, Bluhm F, Dumont, RA, Braun F, Melpomeni F, Riesner K, Prinz G, Hechinger AK, Gerlach UV, Dierbach H, Penack O, Schmitt-Gräff A, Finke J, Weber WA, Zeiser R. Inflammatory neovascularization during graft-versus-host disease is regulated by αv integrin and miR-100.
Blood 121:3307-3318, 2013
Highlighted in Komanduri KV et al. Blood 121:3303-4, 2014
Wilhelm, K, Ganesan J, Müller T, Dürr C, Grimm, M, Beilhack, A, Krempl, CD, Sorichter, S, Gerlach, UV, Jüttner, E, Zerweck, A, Gärtner, F, Pellegatti, P, Di Virgilio, F, Ferrari, D, Kambham, N, Fisch, P, Finke, J, Idzko, M, Zeiser, R. Graft-versus-host disease is enhanced by extracellular adenosine triphosphate activating P2X7R.
Nature Medicine 12: 1434-1438, 2010

Zeiser R, Spyridonidis A, Wasch R, Ihorst G, Grullich C, Bertz H, Finke J. Evaluation of immunomodulatory treatment based on conventional and lineage-specific chimerism analysis in patients with myeloid malignancies after myeloablative allogeneic hematopoietic cell transplantation.
Leukemia 19: 814-821, 2005.
2025
Gottfried Wilhelm Leibniz-Preis 2025 (DFG, Germany)
Donnall Thomas Lecture Prize 2025 (ASTCT, USA)
2024
DKMS Mechtild Harf Science Award 2024 (Glasgow)
2023
ERC Advanced Grant (European Research Council)
2022
Disease prevention award (Deutsche Gesellschaft für Innere Medizin)
2021
Schaefer Award (DAG-HSZT)
2021
CIBSS Award (Cluster of Excellence CIBSS)
2021
Deutscher Krebspreis (Deutsche Krebsgesellschaft)
2020
Science Award of the Berlin Brandenburgische Akademie der Wissenschaften
2019
Paul-Martini-Preis 2019
2017
Richtzenhain-Preis 2016 (Deutsche Krebsforschungszentrum, DKFZ)
2016
ERC Consolidator grant (European Research Council)
2015
Jon J. van Rood Award (European Society for Blood and Marrow Transplantation, EBMT, Istanbul)
2014
Artur Pappenheim Award (German Society of Hematology and Oncology (DGHO) Conference, Hamburg)
2014
Hans Jochem Kolb Award (GvHD/GvL Conference, Regensburg)
2014
Karin Nolte Research Award (SP Internal Medicine Society, Neustadt)
2012
BMT Research Award (German Bone Marrow Transplantation Society, Berlin)
2011
Theodor Frerichs Award (German Society of Internal Medicine, Wiesbaden)
2011
Elected Heisenberg Fellow (German Research Foundation, DFG)
2008
BBMT Editorial Award: Best basic science article of 2007 (ASBMT, San Diego)
2007
Eugen Graetz Research Award, Frbg. University Research Foundation (Freiburg)
2006
ASH Scholar Award of the American Society of Hematology (ASH, Orlando)
2006
Trainee Research Award, American Society of Hematology

The research group of Dr. Florian Ingelfinger, Centre for Translational Cell Research (ZTZ), at the Medical Center – University of Freiburg, Department of Hematology, Oncology and Stem Cell Transplantation, invites applications from
Master Students/Internships (m/f/d)
Starting date: as soon as possible
Project: Deep Generative Modeling of Immune Phenotypes in Acute Myeloid Leukemia
Laboratory Activities
Our lab members are international...
Animal Imaging Advisory Board
The Animal Imaging Advisory Board is a consulting service for preclinical imagingThe small animal imaging advisory board will help the PIs of the CRC1479 initiative to choose the best imaging method to answer the scientific questions posed by their project. BLI, NMR-spectroscopy, MR hyperpolarisation, ultrasound and CT-based imaging will be provided. Each of this modality has some strengths and weaknesses. Therefore it will be challenging for the individual group leaders to decide which animal imaging modality is the best to answer their specific questions. The animal advisory board should be a forum which supports the project coordinators to define the best approach in respect to their specific requests
The imaging advisory board consists of one project coordinator from each modality.
MRI: Dr. Wilfried Reichardt | wilfried.reichardt@uniklinik-freiburg.de
BLI: Prof. Dr. Robert Zeiser | robert.zeiser@uniklinik-freiburg.de
The PI with interest in animal imaging needs to send the application sheet and a short abstract (about half a side) with the description of the request for an imaging application. The three members review it in a bimonthly meeting and give an advice to the applicant.
Table 1: Complementarity of two representative imaging platforms
| Method | Advantage |
| BLI | • Fast and low cost method for living animals • High sensitivity • Evaluating the kinetics of cell division is possible (signal intensity increases) • Small cell populations can be detected (as little as 1000 cells in superficial areas) • The presence of bioluminescence signal indicates that the luc+ cell population is viable • Luciferase can be used as reporter-probe to monitor promoter activity of a gene of interest |
| MRI | • Highest spatial resolution (< 100 µm ) • Directly translational to human application • Combines multi contrast 3D morphological with multi modal functional imaging. (e.g. Diffusion, Flow, fMRI, DCE-MRI and other) • Passive, active and activatable contrast agents possible |
In vivo Bioluminescence Imaging (BLI)
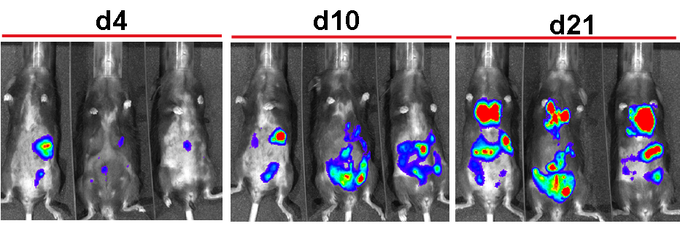
Luciferase-based imaging methods have been recently applied to detect widespread metastasis in different murine tumour models including breast cancer, prostate cancer, ovarian cancer, B cell lymphoma and other entities. BLI is of enormous advantage when small cell populations need to be isolated since it can provide guidance which anatomical regions need to be removed for further analysis. When luc transgenic cells are employed BLI is permissive for cell proliferation analysis since the reporter gene is duplicated upon cell division and thus signal intensity correlates with cell number. Combination of Luc expression and GFP expression in tumor cells allows further purification and analysis of tumor cell by FACS after BLI guided in vivo monitoring and recovery
Nuclear Magnetic Resonance Imaging (MRI)
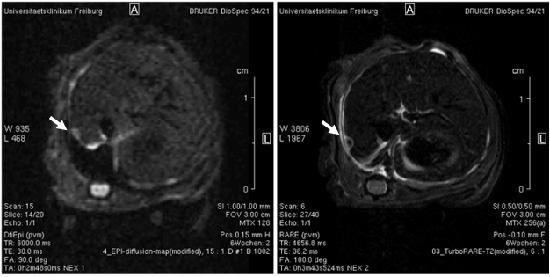
The ability to generate multiple contrasts non invasively and at high resolution including various functional contrasts has given MRI an important role in clinical diagnostic imaging. These techniques have been translated to dedicated animal systems. Small animal MR is therefore already extensively used in preclinical oncology research, and its impact is growing as in addition to morphology and volumetry numerous processes can be quantitatively imaged. Numerous studies have for example shown the use of MR for measurements of perfusion and permeability by of tissue using DCE-MRI. Diffusion MR has been shown to be effective to control tumor response and to monitor apoptosis. 1H-, 31P- and 13C- spectroscopy has been extensively used to observe tumor metabolism. Currently, rapid progress is being made in the development of molecular probes to observe specific receptors as well as migration of iron oxide or 19F labeled cells. Combined application of parametric measurements has been shown to be a promising tool for therapeutic monitoring and imaging phenotyping of animal models.